Cullin4 E3 Ubiquitin Ligases Regulate Male Gonocyte Migration, Proliferation and Blood-Testis Barrier Homeostasis
- PMID: 34685710
- PMCID: PMC8535100
- DOI: 10.3390/cells10102732
Cullin4 E3 Ubiquitin Ligases Regulate Male Gonocyte Migration, Proliferation and Blood-Testis Barrier Homeostasis
Abstract
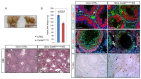
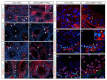
Ubiquitination, an essential posttranslational modification, plays fundamental roles during mammalian spermatogenesis. We previously reported the requirement of two Cullin 4 ubiquitin ligase family genes, Cullin 4a (Cul4a) and Cullin 4b (Cul4b), in murine spermatogenesis. Both genes are required for male fertility despite their distinct functions in different cell populations. Cul4a is required in primary spermatocytes to promote meiosis while Cul4b is required in secondary spermatocytes for spermiogenesis. As the two genes encode proteins that are highly homologous and have overlapping expression in embryonic germ cells, they may compensate for each other during germ cell development. In the present study, we directly address the potential functional redundancy of these two proteins by deleting both Cul4 genes, specifically, in the germ cell lineage during embryonic development, using the germ-cell specific Vasa-Cre line. Conditional double-knockout (dKO) males showed delayed homing and impaired proliferation of gonocytes, and a complete loss of germ cells before the end of the first wave of spermatogenesis. The dKO male germ cell phenotype is much more severe than those observed in either single KO mutant, demonstrating the functional redundancy between the two CUL4 proteins. The dKO mutant also exhibited atypical tight junction structures, suggesting the potential involvement of CUL4 proteins in spermatogonial stem cell (SSC) niche formation and blood-testis-barrier (BTB) maintenance. We also show that deleting Cul4b in both germ and Sertoli cells is sufficient to recapitulate part of this phenotype, causing spermatogenesis defects and drastically reduced number of mature sperms, accompanied by defective tight junctions in the mutant testes. These results indicate the involvement of CUL4B in maintaining BTB integrity.
Keywords: Cullin4; blood-testis barrier; spermatogenesis; ubiquitination.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Muscarinic acetylcholine receptor M5 is involved in spermatogenesis through the modification of cell-cell junctions.Reproduction. 2021 May 27;162(1):47-59. doi: 10.1530/REP-21-0079. Reproduction. 2021. PMID: 33970124 Free PMC article.
-
Temporal maturation of Sertoli cells during the establishment of the cycle of the seminiferous epithelium†.Biol Reprod. 2024 Oct 14;111(4):959-974. doi: 10.1093/biolre/ioae115. Biol Reprod. 2024. PMID: 39077996 Free PMC article.
-
Polyethylene microplastics disrupt focal adhesion kinase (FAK) signaling and sertoli cell metabolism, compromising blood-testis barrier function and spermatogenesis.Toxicology. 2025 Nov;517:154240. doi: 10.1016/j.tox.2025.154240. Epub 2025 Jul 22. Toxicology. 2025. PMID: 40701262
-
Metabolic pathways and male fertility: exploring the role of Sertoli cells in energy homeostasis and spermatogenesis.Am J Physiol Endocrinol Metab. 2025 Jul 1;329(1):E160-E178. doi: 10.1152/ajpendo.00074.2025. Epub 2025 Jun 16. Am J Physiol Endocrinol Metab. 2025. PMID: 40522874 Review.
-
The Black Book of Psychotropic Dosing and Monitoring.Psychopharmacol Bull. 2024 Jul 8;54(3):8-59. Psychopharmacol Bull. 2024. PMID: 38993656 Free PMC article. Review.
Cited by
-
A novel CUL4B gene variant activating Wnt4/β-catenin signal pathway to karyotype 46, XY female with disorders of sex development.Biol Res. 2025 Jan 7;58(1):1. doi: 10.1186/s40659-024-00583-1. Biol Res. 2025. PMID: 39773765 Free PMC article.
-
The Role of microRNAs in the Gonocyte Theory as Target of Malignancy: Looking for Potential Diagnostic Biomarkers.Int J Mol Sci. 2022 Sep 10;23(18):10526. doi: 10.3390/ijms231810526. Int J Mol Sci. 2022. PMID: 36142439 Free PMC article. Review.
-
CRL2LRRC41-Mediated DDX5 Ubiquitination Enhances Interaction with ELAVL1 Preventing NOG mRNA Degradation and Sustaining Proliferation and Migration of Human Spermatogonial Stem Cell-Like Cell Line.BMC Biol. 2025 Aug 7;23(1):247. doi: 10.1186/s12915-025-02363-z. BMC Biol. 2025. PMID: 40775760 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

