Sirtuins as Interesting Players in the Course of HIV Infection and Comorbidities
- PMID: 34685718
- PMCID: PMC8534645
- DOI: 10.3390/cells10102739
Sirtuins as Interesting Players in the Course of HIV Infection and Comorbidities
Abstract
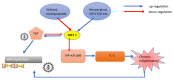
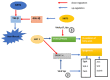
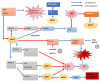
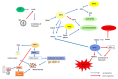
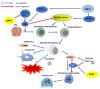
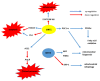
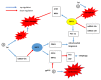
The sirtuins (SIRTs) are a family of enzymes from the group of NAD+-dependent deacetylases. Through the reaction of splitting the acetyl group of various transcription factors and histones they regulate many processes in the organism. The activity of sirtuins is linked to metabolic control, oxidative stress, inflammation and apoptosis, and they also affect the course of viral infections. For this reason, they may participate in the pathogenesis and development of many diseases, but little is known about their role in the course of human immunodeficiency virus (HIV) infection, which is the subject of this review. In the course of HIV infection, comorbidities such as: neurodegenerative disorders, obesity, insulin resistance and diabetes, lipid disorders and cardiovascular diseases, renal and bone diseases developed more frequently and faster compared to the general population. The role of sirtuins in the development of accompanying diseases in the course of HIV infection may also be interesting. There is still a lack of detailed information on this subject. The role of sirtuins, especially SIRT1, SIRT3, SIRT6, are indicated to be of great importance in the course of HIV infection and the development of the abovementioned comorbidities.
Keywords: HAART; HIV; cART; comorbidities; sirtuins.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Serrão R., Piñero C., Velez J., Coutinho D., Maltez F., Lino S., Sarmento e Castro R., Tavares A.P., Pacheco P., Lopes M.J., et al. Non-AIDS-related comorbidities in people living with HIV-1 aged 50 years and older: The AGING POSITIVE study. Int. J. Infect. Dis. 2019;79:94–100. doi: 10.1016/j.ijid.2018.10.011. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

