The Potential Role of Cytokines and Growth Factors in the Pathogenesis of Alzheimer's Disease
- PMID: 34685770
- PMCID: PMC8534363
- DOI: 10.3390/cells10102790
The Potential Role of Cytokines and Growth Factors in the Pathogenesis of Alzheimer's Disease
Abstract
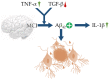
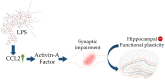
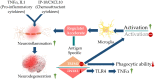
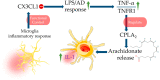
Alzheimer's disease (AD) is one of the most prominent neurodegenerative diseases, which impairs cognitive function in afflicted individuals. AD results in gradual decay of neuronal function as a consequence of diverse degenerating events. Several neuroimmune players (such as cytokines and growth factors that are key players in maintaining CNS homeostasis) turn aberrant during crosstalk between the innate and adaptive immunities. This aberrance underlies neuroinflammation and drives neuronal cells toward apoptotic decline. Neuroinflammation involves microglial activation and has been shown to exacerbate AD. This review attempted to elucidate the role of cytokines, growth factors, and associated mechanisms implicated in the course of AD, especially with neuroinflammation. We also evaluated the propensities and specific mechanism(s) of cytokines and growth factors impacting neuron upon apoptotic decline and further shed light on the availability and accessibility of cytokines across the blood-brain barrier and choroid plexus in AD pathophysiology. The pathogenic and the protective roles of macrophage migration and inhibitory factors, neurotrophic factors, hematopoietic-related growth factors, TAU phosphorylation, advanced glycation end products, complement system, and glial cells in AD and neuropsychiatric pathology were also discussed. Taken together, the emerging roles of these factors in AD pathology emphasize the importance of building novel strategies for an effective therapeutic/neuropsychiatric management of AD in clinics.
Keywords: Alzheimer’s disease; blood brain barrier; brain health; chemokines; cytokines; mild cognitive impairment; neuroinflammation; neurotrophic factors; pathophysiology; therapeutics.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Neuroinflammation: implications for the pathogenesis and molecular diagnosis of Alzheimer's disease.Arch Med Res. 2008 Jan;39(1):1-16. doi: 10.1016/j.arcmed.2007.10.001. Epub 2007 Oct 31. Arch Med Res. 2008. PMID: 18067990 Review.
-
Neuroinflammation in Alzheimer's Disease: The Preventive and Therapeutic Potential of Polyphenolic Nutraceuticals.Adv Protein Chem Struct Biol. 2017;108:33-57. doi: 10.1016/bs.apcsb.2017.02.001. Epub 2017 Mar 22. Adv Protein Chem Struct Biol. 2017. PMID: 28427563 Review.
-
Key role of MIF-related neuroinflammation in neurodegeneration and cognitive impairment in Alzheimer's disease.Mol Med. 2020 Apr 17;26(1):34. doi: 10.1186/s10020-020-00163-5. Mol Med. 2020. PMID: 32303185 Free PMC article.
-
Roles of Cytokines in Alzheimer's Disease.Int J Mol Sci. 2024 May 26;25(11):5803. doi: 10.3390/ijms25115803. Int J Mol Sci. 2024. PMID: 38891990 Free PMC article. Review.
-
Plasma neuronal exosomes serve as biomarkers of cognitive impairment in HIV infection and Alzheimer's disease.J Neurovirol. 2019 Oct;25(5):702-709. doi: 10.1007/s13365-018-0695-4. Epub 2019 Jan 4. J Neurovirol. 2019. PMID: 30610738 Free PMC article. Review.
Cited by
-
Glial Gap Junction Pathology in the Spinal Cord of the 5xFAD Mouse Model of Early-Onset Alzheimer's Disease.Int J Mol Sci. 2022 Dec 9;23(24):15597. doi: 10.3390/ijms232415597. Int J Mol Sci. 2022. PMID: 36555237 Free PMC article.
-
Myelin in Alzheimer's disease: culprit or bystander?Acta Neuropathol Commun. 2023 Mar 31;11(1):56. doi: 10.1186/s40478-023-01554-5. Acta Neuropathol Commun. 2023. PMID: 37004127 Free PMC article. Review.
-
Gallic acid ameliorates LPS-induced memory decline by modulating NF-κB, TNF-α, and Caspase 3 gene expression and attenuating oxidative stress and neuronal loss in the rat hippocampus.Metab Brain Dis. 2024 Nov 18;40(1):12. doi: 10.1007/s11011-024-01441-5. Metab Brain Dis. 2024. PMID: 39556267
-
How the Dietary Saturated/Monounsaturated Fatty Acid Ratio Modulates Brain Function in Older Adults.Nutrients. 2025 May 31;17(11):1897. doi: 10.3390/nu17111897. Nutrients. 2025. PMID: 40507166 Free PMC article. Review.
-
Morphometry and Stiffness of Red Blood Cells-Signatures of Neurodegenerative Diseases and Aging.Int J Mol Sci. 2021 Dec 25;23(1):227. doi: 10.3390/ijms23010227. Int J Mol Sci. 2021. PMID: 35008653 Free PMC article.
References
-
- Bergström P., Agholme L., Nazir F.H., Satir T.M., Toombs J., Wellington H., Strandberg J., Bontell T.O., Kvartsberg H., Holmström M., et al. Amyloid precursor protein expression and processing are differentially regulated during cortical neuron differentiation. Sci. Rep. 2016;6:29200. doi: 10.1038/srep29200. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

