Antiangiogenic antibody BD0801 combined with immune checkpoint inhibitors achieves synergistic antitumor activity and affects the tumor microenvironment
- PMID: 34686154
- PMCID: PMC8539826
- DOI: 10.1186/s12885-021-08859-5
Antiangiogenic antibody BD0801 combined with immune checkpoint inhibitors achieves synergistic antitumor activity and affects the tumor microenvironment
Abstract
Background: Signaling through VEGF/VEGFR induces cancer angiogenesis and affects immune cells. An increasing number of studies have recently focused on combining anti-VEGF/VEGFR agents and immune checkpoint inhibitors (ICIs) to treat cancer in preclinical and clinical settings. BD0801 is a humanized rabbit anti-VEGF monoclonal antibody in the clinical development stage.
Methods: In this study, the anti-cancer activities of BD0801 and its potential synergistic anti-tumor effects when combined with different immunotherapies were assessed by using in vitro assays and in vivo tumor models. Ex vivo studies were conducted to reveal the possible mechanisms of actions (MOA) underlying the tumor microenvironment modification.
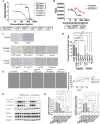
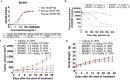
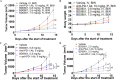
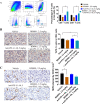
Results: BD0801 showed more potent antitumor activity than bevacizumab, reflected by stronger blockade of VEGF/VEGFR binding and enhanced inhibitory effects on human umbilical vein endothelial cells (HUVECs). BD0801 exhibited dose-dependent tumor growth inhibitory activities in xenograft and murine syngeneic tumor models. Notably, combining BD0801 with either anti-PD-1 or anti-PD-L1 antibodies showed synergistic antitumor efficacy in both lung and colorectal cancer mouse models. Furthermore, the mechanistic studies suggested that the MOA of the antitumor synergy involves improved tumor vasculature normalization and enhanced T-cell mediated immunity, including increased tumor infiltration of CD8+ and CD4+ T cells and reduced double-positive CD8+PD-1+ T cells.
Conclusions: These data provide a solid rationale for combining antiangiogenic agents with immunotherapy for cancer treatment and support further clinical development of BD0801 in combination with ICIs.
Keywords: Anti-VEGF monoclonal antibody; Antitumor synergy; Combination treatment; Immune checkpoint blockade; Tumor microenvironment.
© 2021. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

