Monitoring mitochondrial calcium and metabolism in the beating MCU-KO heart
- PMID: 34686324
- PMCID: PMC10461605
- DOI: 10.1016/j.celrep.2021.109846
Monitoring mitochondrial calcium and metabolism in the beating MCU-KO heart
Abstract
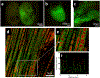
Optical methods for measuring intracellular ions including Ca2+ revolutionized our understanding of signal transduction. However, these methods are not extensively applied to intact organs due to issues including inner filter effects, motion, and available probes. Mitochondrial Ca2+ is postulated to regulate cell energetics and death pathways that are best studied in an intact organ. Here, we develop a method to optically measure mitochondrial Ca2+ and demonstrate its validity for mitochondrial Ca2+ and metabolism using hearts from wild-type mice and mice with germline knockout of the mitochondria calcium uniporter (MCU-KO). We previously reported that germline MCU-KO hearts do not show an impaired response to adrenergic stimulation. We find that these MCU-KO hearts do not take up Ca2+, consistent with no alternative Ca2+ uptake mechanisms in the absence of MCU. This approach can address the role of mitochondrial Ca2+ to the myriad of functions attributed to alterations in mitochondrial Ca2+.
Keywords: calcium; heart; isoproterenol; mitochondria; spectroscopy.
Published by Elsevier Inc.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures
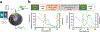
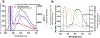
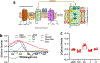



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

