Ultra-selective molecular-sieving gas separation membranes enabled by multi-covalent-crosslinking of microporous polymer blends
- PMID: 34686671
- PMCID: PMC8536662
- DOI: 10.1038/s41467-021-26379-5
Ultra-selective molecular-sieving gas separation membranes enabled by multi-covalent-crosslinking of microporous polymer blends
Abstract
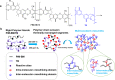
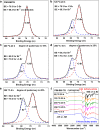
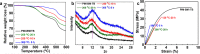
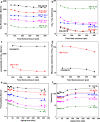
High-performance membranes exceeding the conventional permeability-selectivity upper bound are attractive for advanced gas separations. In the context microporous polymers have gained increasing attention owing to their exceptional permeability, which, however, demonstrate a moderate selectivity unfavorable for separating similarly sized gas mixtures. Here we report an approach to designing polymeric molecular sieve membranes via multi-covalent-crosslinking of blended bromomethyl polymer of intrinsic microporosity and Tröger's base, enabling simultaneously high permeability and selectivity. Ultra-selective gas separation is achieved via adjusting reaction temperature, reaction time and the oxygen concentration with occurrences of polymer chain scission, rearrangement and thermal oxidative crosslinking reaction. Upon a thermal treatment at 300 °C for 5 h, membranes exhibit an O2/N2, CO2/CH4 and H2/CH4 selectivity as high as 11.1, 154.5 and 813.6, respectively, transcending the state-of-art upper bounds. The design strategy represents a generalizable approach to creating molecular-sieving polymer membranes with enormous potentials for high-performance separation processes.
© 2021. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures




References
LinkOut - more resources
Full Text Sources

