Computational modeling of seizure spread on a cortical surface
- PMID: 34686937
- PMCID: PMC8818012
- DOI: 10.1007/s10827-021-00802-8
Computational modeling of seizure spread on a cortical surface
Abstract
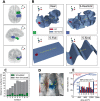
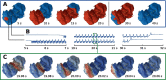
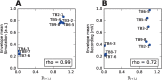
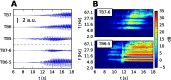
In the field of computational epilepsy, neural field models helped to understand some large-scale features of seizure dynamics. These insights however remain on general levels, without translation to the clinical settings via personalization of the model with the patient-specific structure. In particular, a link was suggested between epileptic seizures spreading across the cortical surface and the so-called theta-alpha activity (TAA) pattern seen on intracranial electrographic signals, yet this link was not demonstrated on a patient-specific level. Here we present a single patient computational study linking the seizure spreading across the patient-specific cortical surface with a specific instance of the TAA pattern recorded in the patient. Using the realistic geometry of the cortical surface we perform the simulations of seizure dynamics in The Virtual Brain platform, and we show that the simulated electrographic signals qualitatively agree with the recorded signals. Furthermore, the comparison with the simulations performed on surrogate surfaces reveals that the best quantitative fit is obtained for the real surface. The work illustrates how the patient-specific cortical geometry can be utilized in The Virtual Brain for personalized model building, and the importance of such approach.
Keywords: Computational modeling; Epilepsy; Seizure propagation.
© 2021. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures




Similar articles
-
Criticality in probabilistic models of spreading dynamics in brain networks: Epileptic seizures.PLoS Comput Biol. 2023 Feb 7;19(2):e1010852. doi: 10.1371/journal.pcbi.1010852. eCollection 2023 Feb. PLoS Comput Biol. 2023. PMID: 36749796 Free PMC article.
-
Evidence for spreading seizure as a cause of theta-alpha activity electrographic pattern in stereo-EEG seizure recordings.PLoS Comput Biol. 2021 Feb 26;17(2):e1008731. doi: 10.1371/journal.pcbi.1008731. eCollection 2021 Feb. PLoS Comput Biol. 2021. PMID: 33635864 Free PMC article.
-
Virtual epilepsy patient cohort: Generation and evaluation.PLoS Comput Biol. 2025 Apr 11;21(4):e1012911. doi: 10.1371/journal.pcbi.1012911. eCollection 2025 Apr. PLoS Comput Biol. 2025. PMID: 40215461 Free PMC article.
-
Computational modeling of whole-brain dynamics: a review of neurosurgical applications.J Neurosurg. 2023 Jun 23;140(1):218-230. doi: 10.3171/2023.5.JNS23250. Print 2024 Jan 1. J Neurosurg. 2023. PMID: 37382356 Review.
-
Multi-scale modelling of the epileptic brain: advantages of computational therapy exploration.J Neural Eng. 2024 Apr 25;21(2). doi: 10.1088/1741-2552/ad3eb4. J Neural Eng. 2024. PMID: 38621378 Review.
Cited by
-
Extracting Dynamical Understanding From Neural-Mass Models of Mouse Cortex.Front Comput Neurosci. 2022 Apr 25;16:847336. doi: 10.3389/fncom.2022.847336. eCollection 2022. Front Comput Neurosci. 2022. PMID: 35547660 Free PMC article.
-
Criticality in probabilistic models of spreading dynamics in brain networks: Epileptic seizures.PLoS Comput Biol. 2023 Feb 7;19(2):e1010852. doi: 10.1371/journal.pcbi.1010852. eCollection 2023 Feb. PLoS Comput Biol. 2023. PMID: 36749796 Free PMC article.
-
Modeling for neurosurgical laser interstitial thermal therapy with and without intracranial recording electrodes.Curr Res Neurobiol. 2024 Sep 15;7:100139. doi: 10.1016/j.crneur.2024.100139. eCollection 2024. Curr Res Neurobiol. 2024. PMID: 39347540 Free PMC article.
-
Localizing targets for neuromodulation in drug-resistant epilepsy using intracranial EEG and computational model.Front Physiol. 2022 Oct 20;13:1015838. doi: 10.3389/fphys.2022.1015838. eCollection 2022. Front Physiol. 2022. PMID: 36338480 Free PMC article.
-
The role of epidemic spreading in seizure dynamics and epilepsy surgery.Netw Neurosci. 2023 Jun 30;7(2):811-843. doi: 10.1162/netn_a_00305. eCollection 2023. Netw Neurosci. 2023. PMID: 37397878 Free PMC article.
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

