Intercontinental collaboration in clinical trials for children and adolescents with cancer-A systematic review by ACCELERATE
- PMID: 34687165
- PMCID: PMC8633236
- DOI: 10.1002/cam4.4356
Intercontinental collaboration in clinical trials for children and adolescents with cancer-A systematic review by ACCELERATE
Abstract
Background: Since pediatric cancer drug development is a global enterprise, we sought to provide an overview of the landscape of intercontinental clinical trials in pediatric oncology opened over the last decade.
Methods: ClinicalTrials.gov was systematically searched to identify all clinical therapeutic trials which opened between 2010 and 2020 and recruited pediatric patients (<18 years) with cancer.
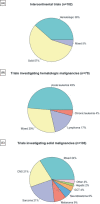
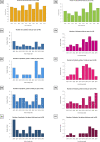
Results: Over the last 10 years, 295 (8.7%) of 3383 therapeutic pediatric cancer trials were international and 182 (5.4%) were intercontinental. Most intercontinental trials were phase-1 or 2, with 25% late-phase, 65% were sponsored by industry, and North America was involved in 92%. Industry-sponsored proportionally more phase-1 trials than academia (41% vs. 25%); conversely, academia sponsored more phase-2 and late-phase trials (39% and 31% vs. 36% and 21%, respectively) (p = 0.020). North America-Europe collaboration was predominantly industry sponsored as opposed to North America-Oceania and Europe-Oceania collaboration, more frequently academic (p < 0.0001). Most late-phase trials (18/20, 90%) focusing on pediatric malignancies were conducted by academic sponsors and 10 of these were conducted by Children's Oncology Group (COG)/National Cancer Institute in the United States and Oceania. There was no significant increase over time of intercontinental trials and a trend for a reduction in academic trials.
Conclusions: Despite the relative rarity of childhood malignancies, especially within molecular subtypes, only 5.4% of pediatric cancer trials were intercontinental. The number of intercontinental trials remains small, with no significant increase over the last decade. The ACCELERATE International Collaboration Working Group aims to identify existing hurdles and propose solutions to improve intercontinental collaboration in clinical research for the benefit of children and adolescents with cancer.
Keywords: adolescent cancer; childhood cancer; clinical research; clinical trials; drug development; international collaboration; rare diseases.
© 2021 The Authors. Cancer Medicine published by John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Gatta G, Botta L, Rossi S, et al. Childhood cancer survival in Europe 1999–2007: results of EUROCARE‐5—a population‐based study. Lancet Oncol. 2014;15:35‐47. - PubMed
-
- Trama A, Botta L, Foschi R, et al. Survival of European adolescents and young adults diagnosed with cancer in 2000–07: population‐based data from EUROCARE‐5. Lancet Oncol. 2016;17:896‐906. - PubMed
-
- Noone AM, Howlader N, Krapcho M, et al. SEER cancer statistics review, 1975–2015. National Cancer Institute; 2018. Accessed September 3, 2018. https://seer.cancer.gov/csr/1975_2015/
-
- The European Society for Paediatric Oncology : The SIOPE strategic plan; a European Cancer Plan for Children and Adolescents. 1st ed. SIOPE; 2015. Accessed October 15, 2021. https://worldspanmedia.s3‐eu‐west‐1.amazonaws.com/media/siope/PDF/the‐si...
-
- Vassal G, Zwaan CM, Ashley D, et al. New drugs for children and adolescents with cancer: the need for novel development pathways. Lancet Oncol. 2013;14:e117‐e124. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

