Evaluation of LL-37 in healing of hard-to-heal venous leg ulcers: A multicentric prospective randomized placebo-controlled clinical trial
- PMID: 34687253
- PMCID: PMC9298190
- DOI: 10.1111/wrr.12977
Evaluation of LL-37 in healing of hard-to-heal venous leg ulcers: A multicentric prospective randomized placebo-controlled clinical trial
Abstract
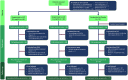
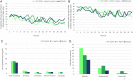
Many patients with venous leg ulcers do not reach complete healing with compression treatment alone, which is current standard care. This clinical trial HEAL LL-37 was a phase IIb double-blind, randomized, placebo-controlled study, with the aim to evaluate the efficacy and safety of a new drug LL-37 for topical administration, in combination with compression therapy, in 148 patients suffering from hard-to-heal venous leg ulcers. The study had three arms, consisting of two groups treated with LL-37 at concentrations of 0.5 or 1.6 mg/mL, and a placebo cohort. Patients had a mean age of 67.6 years, a median ulcer duration of 20.3 months, and a mean wound size at the time of randomization of 11.6 cm2 . Efficacy analysis performed on the full study population did not identify any significant improvement in healing in patients treated with LL-37 as compared with the placebo. In contrast, a post hoc analysis revealed statistically significant improvement with LL-37 treatment in several interrelated healing parameters in the subgroup of patients with large target wounds (a wound area of at least 10 cm2 at randomization), which is a known negative prognostic factor for healing. The study drug was well tolerated and safe in both dose strengths. In summary, this clinical trial did not detect any significant differences in healing of venous lower leg ulcers in the entire study cohort comparing patients treated with LL-37 versus placebo. A subgroup analysis provided an interesting observation that LL-37 could offer a treatment benefit in patients with large ulcers, exigently warranting a further study adequately powered to statistically assess the treatment outcome in this patient group.
Keywords: LL-37; phase II clinical trial; venous leg ulcers; wound healing.
© 2021 Promore Pharma AB. Wound Repair and Regeneration published by Wiley Periodicals LLC on behalf of The Wound Healing Society.
Conflict of interest statement
At the time of the investigation, Margit Mahlapuu, Jakob Björk, and Jonas Ekblom were employees at Promore Pharma AB. Jonas Ekblom is a minority shareholder at Promore Pharma AB. Promore Pharma AB is developing LL‐37 under the non‐proprietary name ropocamptide.
Figures

References
-
- Graham ID, Harrison MB, Nelson EA, Lorimer K, Fisher A. Prevalence of lower‐limb ulceration: a systematic review of prevalence studies. Adv Skin Wound Care. 2003;16(6):305‐316. - PubMed
-
- Franks PJ, Barker J, Collier M, et al. Management of patients with venous leg ulcers: challenges and current best practice. J Wound Care. 2016;25:S1‐S67. - PubMed
-
- Joaquim FL, Silva R, Garcia‐Caro MP, Cruz‐Quintana F, Pereira ER. Impact of venous ulcers on patients' quality of life: an integrative review. Rev Bras Enferm. 2018;71(4):2021‐2029. - PubMed
-
- Rivas‐Santiago B, Rivas Santiago CE, Castaneda‐Delgado JE, Leon‐Contreras JC, Hancock RE, Hernandez‐Pando R. Activity of LL‐37, CRAMP and antimicrobial peptide‐derived compounds E2, E6 and CP26 against Mycobacterium tuberculosis. Int J Antimicrob Agents. 2013;41(2):143‐148. - PubMed

