SARS-CoV-2 Spike Protein Disrupts Blood-Brain Barrier Integrity via RhoA Activation
- PMID: 34687399
- PMCID: PMC8536479
- DOI: 10.1007/s11481-021-10029-0
SARS-CoV-2 Spike Protein Disrupts Blood-Brain Barrier Integrity via RhoA Activation
Abstract
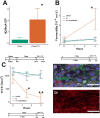
The SARS-CoV-2 spike protein has been shown to disrupt blood-brain barrier (BBB) function, but its pathogenic mechanism of action is unknown. Whether angiotensin converting enzyme 2 (ACE2), the viral binding site for SARS-CoV-2, contributes to the spike protein-induced barrier disruption also remains unclear. Here, a 3D-BBB microfluidic model was used to interrogate mechanisms by which the spike protein may facilitate barrier dysfunction. The spike protein upregulated the expression of ACE2 in response to laminar shear stress. Moreover, interrogating the role of ACE2 showed that knock-down affected endothelial barrier properties. These results identify a possible role of ACE2 in barrier homeostasis. Analysis of RhoA, a key molecule in regulating endothelial cytoskeleton and tight junction complex dynamics, reveals that the spike protein triggers RhoA activation. Inhibition of RhoA with C3 transferase rescues its effect on tight junction disassembly. Overall, these results indicate a possible means by which the engagement of SARS-CoV-2 with ACE2 facilitates disruption of the BBB via RhoA activation. Understanding how SARS-CoV-2 dysregulates the BBB may lead to strategies to prevent the neurological deficits seen in COVID-19 patients.
Keywords: Blood–brain barrier; Fluid shear stress; Mechanotransduction; RhoA; SARS-CoV-2.
© 2021. The Author(s), under exclusive licence to Springer Science+Business Media, LLC, part of Springer Nature.
Conflict of interest statement
The authors report no conflicts of interest.
Figures


References
-
- Abedi F, Hayes AW, Reiter R, Karimi G (2020a) Acute lung injury: The therapeutic role of Rho kinase inhibitors. Pharmacol Res 155:104736 - PubMed
-
- Acharya NK, Goldwaser EL, Forsberg MM, Godsey GA, Johnson CA, Sarkar A, et al. Sevoflurane and Isoflurane induce structural changes in brain vascular endothelial cells and increase blood-brain barrier permeability: Possible link to postoperative delirium and cognitive decline. Brain Res. 2015;1620:29–41. doi: 10.1016/j.brainres.2015.04.054. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

