Pulmonary fibrosis from molecular mechanisms to therapeutic interventions: lessons from post-COVID-19 patients
- PMID: 34687672
- PMCID: PMC8546906
- DOI: 10.1016/j.bcp.2021.114812
Pulmonary fibrosis from molecular mechanisms to therapeutic interventions: lessons from post-COVID-19 patients
Abstract
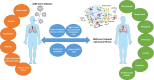
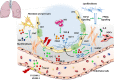
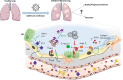
Pulmonary fibrosis (PF) is characterised by several grades of chronic inflammation and collagen deposition in the interalveolar space and is a hallmark of interstitial lung diseases (ILDs). Recently, infectious agents have emerged as driving causes for PF development; however, the role of viral/bacterial infections in the initiation and propagation of PF is still debated. In this context, the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the virus responsible for the current coronavirus disease 2019 (COVID-19) pandemic, has been associated with acute respiratory distress syndrome (ARDS) and PF development. Although the infection by SARS-CoV-2 can be eradicated in most cases, the development of fibrotic lesions cannot be precluded; furthermore, whether these lesions are stable or progressive fibrotic events is still unknown. Herein, an overview of the main molecular mechanisms driving the fibrotic process together with the currently approved and newly proposed therapeutic solutions was given. Then, the most recent data that emerged from post-COVID-19 patients was discussed, in order to compare PF and COVID-19-dependent PF, highlighting shared and specific mechanisms. A better understanding of PF aetiology is certainly needed, also to develop effective therapeutic strategies and COVID-19 pathology is offering one more chance to do it. Overall, the work reported here could help to define new approaches for therapeutic intervention in the diversity of the ILD spectrum.
Keywords: Interstitial lung disease; Myofibroblast; Pulmonary fibrosis; SARS-CoV-2 severe acute respiratory syndrome coronavirus 2.
Copyright © 2021 Elsevier Inc. All rights reserved.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures

Similar articles
-
Pulmonary fibrosis in COVID-19: mechanisms, consequences and targets.QJM. 2023 Oct 6;116(9):750-754. doi: 10.1093/qjmed/hcad092. QJM. 2023. PMID: 37191984 Review.
-
[Pulmonary fibrosis in patients with COVID-19: A review].Ter Arkh. 2022 Dec 26;94(11):1333-1339. doi: 10.26442/00403660.2022.11.201943. Ter Arkh. 2022. PMID: 37167174 Review. Russian.
-
Cellular and Molecular Mechanism of Pulmonary Fibrosis Post-COVID-19: Focus on Galectin-1, -3, -8, -9.Int J Mol Sci. 2022 Jul 26;23(15):8210. doi: 10.3390/ijms23158210. Int J Mol Sci. 2022. PMID: 35897786 Free PMC article. Review.
-
Post-COVID pulmonary sequelae: Mechanisms and potential targets to reduce persistent fibrosis.Pharmacol Ther. 2025 Aug;272:108891. doi: 10.1016/j.pharmthera.2025.108891. Epub 2025 May 28. Pharmacol Ther. 2025. PMID: 40447142 Review.
-
Overactive Epidermal Growth Factor Receptor Signaling Leads to Increased Fibrosis after Severe Acute Respiratory Syndrome Coronavirus Infection.J Virol. 2017 May 26;91(12):e00182-17. doi: 10.1128/JVI.00182-17. Print 2017 Jun 15. J Virol. 2017. PMID: 28404843 Free PMC article.
Cited by
-
COVID-19: A threat to the respiratory system.Int J Immunopathol Pharmacol. 2024 Jan-Dec;38:3946320241310307. doi: 10.1177/03946320241310307. Int J Immunopathol Pharmacol. 2024. PMID: 39716038 Free PMC article. Review.
-
Benefits of Early Systemic Corticosteroid in Clinical Deterioration of Post-COVID-19 Interstitial Lung Disease.Tuberc Respir Dis (Seoul). 2022 Oct;85(4):358-360. doi: 10.4046/trd.2022.0072. Epub 2022 Aug 11. Tuberc Respir Dis (Seoul). 2022. PMID: 35950320 Free PMC article. No abstract available.
-
The effectiveness of glucocorticoid treatment in post-COVID-19 pulmonary involvement.Pneumonia (Nathan). 2024 Feb 5;16(1):2. doi: 10.1186/s41479-023-00123-7. Pneumonia (Nathan). 2024. PMID: 38311783 Free PMC article.
-
Differential Transcriptomic Signatures of Small Airway Cell Cultures Derived from IPF and COVID-19-Induced Exacerbation of Interstitial Lung Disease.Cells. 2023 Oct 21;12(20):2501. doi: 10.3390/cells12202501. Cells. 2023. PMID: 37887346 Free PMC article.
-
CT findings in "Post-Covid": residua from acute pneumonia or "Post-Covid-ILD"?Sarcoidosis Vasc Diffuse Lung Dis. 2023 Jun 29;40(2):e2023024. doi: 10.36141/svdld.v40i2.13983. Sarcoidosis Vasc Diffuse Lung Dis. 2023. PMID: 37382073 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

