Evidence for retained spike-binding and neutralizing activity against emerging SARS-CoV-2 variants in serum of COVID-19 mRNA vaccine recipients
- PMID: 34688034
- PMCID: PMC8527879
- DOI: 10.1016/j.ebiom.2021.103626
Evidence for retained spike-binding and neutralizing activity against emerging SARS-CoV-2 variants in serum of COVID-19 mRNA vaccine recipients
Abstract
Background: Highly efficacious vaccines against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) have been developed. However, the emergence of viral variants that are more infectious than the earlier SARS-CoV-2 strains is concerning. Several of these viral variants have the potential to partially escape neutralizing antibody responses, warranting continued immune-monitoring.
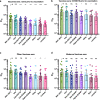
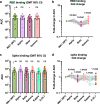
Methods: We used a panel of 30 post-mRNA vaccination sera to determine neutralization and RBD and spike binding activity against a number of emerging viral variants. The virus neutralization was determined using authentic SARS-CoV-2 clinical isolates in an assay format that mimics physiological conditions.
Findings: We tested seven currently circulating viral variants of concern/interest, including the three Iota sublineages, Alpha (E484K), Beta, Delta and Lambda in neutralization assays. We found only small decreases in neutralization against Iota and Delta. The reduction was stronger against a sub-variant of Lambda, followed by Beta and Alpha (E484K). Lambda is currently circulating in parts of Latin America and was detected in Germany, the US and Israel. Of note, reduction in a receptor binding domain and spike binding assay that also included Gamma, Kappa and A.23.1 was negligible.
Interpretation: Taken together, these findings suggest that mRNA SARS-CoV-2 vaccines may remain effective against these viral variants of concern/interest and that spike binding antibody tests likely retain specificity in the face of evolving SARS-CoV-2 diversity.
Funding: This work is part of the PARIS/SPARTA studies funded by the NIAID Collaborative Influenza Vaccine Innovation Centers (CIVIC) contract 75N93019C00051. In addition, this work was also partially funded by the Centers of Excellence for Influenza Research and Surveillance (CEIRS, contract # HHSN272201400008C), the JPB Foundation, the Open Philanthropy Project (research grant 2020-215611 (5384), by anonymous donors and by the Serological Sciences Network (SeroNet) in part with Federal funds from the National Cancer Institute, National Institutes of Health, under Contract No. 75N91019D00024, Task Order No. 75N91020F00003.
Keywords: Antibodies; COVID-19; COVID-19 mRNA vaccines; Neutralization activity; SARS-CoV-2 variants; VoC.
Copyright © 2021 The Authors. Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of Competing Interest The Icahn School of Medicine at Mount Sinai has filed patent applications relating to SARS-CoV-2 serological assays and NDV-based SARS-CoV-2 vaccines which list Florian Krammer as co-inventor. Viviana Simon and Fatima Amanat are also listed on the serological assay patent application as co-inventors. Mount Sinai has spun out a company, Kantaro, to market serological tests for SARS-CoV-2. Florian Krammer has consulted for Merck and Pfizer (before 2020), and is currently consulting for Pfizer, Seqirus and Avimex. The Krammer laboratory is also collaborating with Pfizer on animal models of SARS-CoV-2.
Figures


References
-
- Krammer F. SARS-CoV-2 vaccines in development. Nature. 2020;586(7830):516–527. - PubMed
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

