SARS-COV-2 vaccine: first-month results of a six-month follow-up study
- PMID: 34688238
- PMCID: PMC10734823
- DOI: 10.3906/sag-2106-63
SARS-COV-2 vaccine: first-month results of a six-month follow-up study
Abstract
Background: Various COVID-19 vaccines are being developed around the world. Important questions to be answered regarding vaccines are efficacy, safety, and whether antibodies are protective when used in different communities. This study aimed to determine seroconversion rates of the inactivated SARS-CoV-2 vaccine in healthcare workers in a hospital and short-term adverse events due to the vaccine.
Methods: The study carried out in Çukurova University, Turkey, comprised of 282 healthcare workers who received two doses of the inactivated SARS-CoV-2 vaccine administered in two 3 µg doses, 28 days apart. On day 28, after the second dose, antiS-RBD IgG and total anti-spike and anti-nucleocapsid IgM and IgG antibodies against SARS-CoV-2 were detected by using in vitro chemiluminescence immunoassay method.
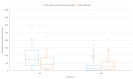
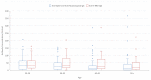
Results: The mean age of participants was 39.06±10.65 (min=21, max=65) with 43.6% males and 56.4% females. On day 28, after the second dose, the seroconversion rates were found to be 92.9% for total anti-spike and anti-nucleocapsid IgG and 15.2% for IgM and 98.2% for anti-S-RBD IgG antibodies and having natural COVID-19 prior to vaccination, age and comorbidity were found to be significant factors for immunogenicity. The incidence of at least one adverse event was found as 29.8% after the first dose and 24.1% after the second dose, with the most common adverse events of having pain at the injection site, weakness, fatigue, and headache.
Keywords: SARS-COV-2; adverse events; immunisation; seroconversion; vaccine.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

