Does infection with or vaccination against SARS-CoV-2 lead to lasting immunity?
- PMID: 34688434
- PMCID: PMC8530467
- DOI: 10.1016/S2213-2600(21)00407-0
Does infection with or vaccination against SARS-CoV-2 lead to lasting immunity?
Abstract
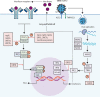
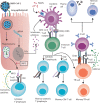
Many nations are pursuing the rollout of SARS-CoV-2 vaccines as an exit strategy from unprecedented COVID-19-related restrictions. However, the success of this strategy relies critically on the duration of protective immunity resulting from both natural infection and vaccination. SARS-CoV-2 infection elicits an adaptive immune response against a large breadth of viral epitopes, although the duration of the response varies with age and disease severity. Current evidence from case studies and large observational studies suggests that, consistent with research on other common respiratory viruses, a protective immunological response lasts for approximately 5-12 months from primary infection, with reinfection being more likely given an insufficiently robust primary humoral response. Markers of humoral and cell-mediated immune memory can persist over many months, and might help to mitigate against severe disease upon reinfection. Emerging data, including evidence of breakthrough infections, suggest that vaccine effectiveness might be reduced significantly against emerging variants of concern, and hence secondary vaccines will need to be developed to maintain population-level protective immunity. Nonetheless, other interventions will also be required, with further outbreaks likely to occur due to antigenic drift, selective pressures for novel variants, and global population mobility.
Copyright © 2021 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Declaration of interests GM was, at the time of writing, a research analyst for the Infectious Disease Modelling Team at the Joint Biosecurity Centre (JBC) of the UK Government Department of Health and Social Care. TH was, at the time of writing, a research analyst for National Alerting and Assessment at the JBC. TW leads the Infectious Disease Modelling Team at the JBC. AJ is a senior data scientist at the Department of Health and Social Care. NG is a senior public health consultant for emergency response at Public Health England. The authors declare no competing interests in relation to the content of this Personal View.
Figures

Comment in
-
Waning immunity to SARS-CoV-2: implications for vaccine booster strategies.Lancet Respir Med. 2021 Dec;9(12):1356-1358. doi: 10.1016/S2213-2600(21)00458-6. Epub 2021 Oct 21. Lancet Respir Med. 2021. PMID: 34688435 Free PMC article. No abstract available.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

