Upregulation of type 1 conventional dendritic cells implicates antigen cross-presentation in multisystem inflammatory syndrome
- PMID: 34688775
- PMCID: PMC8530782
- DOI: 10.1016/j.jaci.2021.10.015
Upregulation of type 1 conventional dendritic cells implicates antigen cross-presentation in multisystem inflammatory syndrome
Erratum in
-
Corrigendum.J Allergy Clin Immunol. 2023 May;151(5):1414. doi: 10.1016/j.jaci.2023.03.003. J Allergy Clin Immunol. 2023. PMID: 37149373 Free PMC article. No abstract available.
Abstract
Background: Multisystem inflammatory syndrome in children (MIS-C) is an acute, febrile, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)-associated syndrome, often with cardiohemodynamic dysfunction. Insight into mechanism of disease is still incomplete.
Objective: Our objective was to analyze immunologic features of MIS-C patients compared to febrile controls (FC).
Methods: MIS-C patients were defined by narrow criteria, including having evidence of cardiohemodynamic involvement and no macrophage activation syndrome. Samples were collected from 8 completely treatment-naive patients with MIS-C (SARS-CoV-2 serology positive), 3 patients with unclassified MIS-C-like disease (serology negative), 14 FC, and 5 MIS-C recovery (RCV). Three healthy controls (HCs) were used for comparisons of normal range. Using spectral flow cytometry, we assessed 36 parameters in antigen-presenting cells (APCs) and 29 in T cells. We used biaxial analysis and uniform manifold approximation and projection (UMAP).
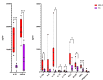
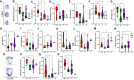
Results: Significant elevations in cytokines including CXCL9, M-CSF, and IL-27 were found in MIS-C compared to FC. Classic monocytes and type 2 dendritic cells (DCs) were downregulated (decreased CD86, HLA-DR) versus HCs; however, type 1 DCs (CD11c+CD141+CLEC9A+) were highly activated in MIS-C patients versus FC, expressing higher levels of CD86, CD275, and atypical conventional DC markers such as CD64, CD115, and CX3CR1. CD169 and CD38 were upregulated in multiple monocyte subtypes. CD56dim/CD57-/KLRGhi/CD161+/CD38- natural killer (NK) cells were a unique subset in MIS-C versus FC without macrophage activation syndrome.
Conclusion: Orchestrated by complex cytokine signaling, type 1 DC activation and NK dysregulation are key features in the pathophysiology of MIS-C. NK cell findings may suggest a relationship with macrophage activation syndrome, while type 1 DC upregulation implies a role for antigen cross-presentation.
Keywords: CLEC9A; Kawasaki disease (KD); Multisystem inflammatory syndrome in children (MIS-C); NK cell cytotoxicity; antigen cross-presentation; dendritic cells.
Copyright © 2021 American Academy of Allergy, Asthma & Immunology. Published by Elsevier Inc. All rights reserved.
Figures




References
-
- Bautista-Rodriguez C., Sanchez-de-Toledo J., Clark B.C., Herberg J., Bajolle F., Randanne P.C., et al. Multisystem inflammatory syndrome in children: an international survey. Pediatrics. 2021;147 e2020024554. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

