The adaptive aging brain
- PMID: 34689041
- PMCID: PMC8901453
- DOI: 10.1016/j.conb.2021.09.009
The adaptive aging brain
Abstract
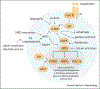
The aging brain is shaped by many structural and functional alterations. Recent cross-disciplinary efforts have uncovered powerful and integrated adaptive mechanisms that promote brain health and prevent functional decline during aging. Here, we review some of the most robust adaptive mechanisms and how they can be engaged to protect, and restore the aging brain.
Copyright © 2021 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Conflict of interest statement
Nothing declared.
Figures



References
-
- James BD, Bennett DA: Causes and patterns of dementia: an update in the era of redefining Alzheimer’s disease. Annu Rev Publ Health 2019, 40:65–84. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

