Somatosensory dysfunction is masked by variable cognitive deficits across patients on the Alzheimer's disease spectrum
- PMID: 34689085
- PMCID: PMC8550984
- DOI: 10.1016/j.ebiom.2021.103638
Somatosensory dysfunction is masked by variable cognitive deficits across patients on the Alzheimer's disease spectrum
Abstract
Background: Alzheimer's disease (AD) is generally thought to spare primary sensory function; however, such interpretations have drawn from a literature that has rarely taken into account the variable cognitive declines seen in patients with AD. As these cognitive domains are now known to modulate cortical somatosensory processing, it remains possible that abnormalities in somatosensory function in patients with AD have been suppressed by neuropsychological variability in previous research.
Methods: In this study, we combine magnetoencephalographic (MEG) brain imaging during a paired-pulse somatosensory gating task with an extensive battery of neuropsychological tests to investigate the influence of cognitive variability on estimated differences in somatosensory function between biomarker-confirmed patients on the AD spectrum and cognitively-normal older adults.
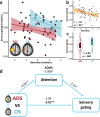
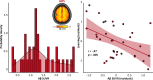
Findings: We show that patients on the AD spectrum exhibit largely non-significant differences in somatosensory function when cognitive variability is not considered (p-value range: .020-.842). However, once attention and processing speed abilities are considered, robust differences in gamma-frequency somatosensory response amplitude (p < .001) and gating (p = .004) emerge, accompanied by significant statistical suppression effects.
Interpretation: These findings suggest that patients with AD exhibit insults to functional somatosensory processing in primary sensory cortices, but these effects are masked by variability in cognitive decline across individuals.
Funding: National Institutes of Health, USA; Fremont Area Alzheimer's Fund, USA.
Keywords: Amyloid-β; Gamma oscillations; Magnetoencephalography; Neuropsychology; Sensory gating.
Copyright © 2021 The Authors. Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of Competing Interest All authors declare no conflicts of interest. Dr. Murman reported receiving grants from Green Valley Pharmaceuticals, Functional Neuromodulation, Roche, and Eli Lilly and Co. and serving on an advisory board for Biogen. Dr. Wilson reported serving as a board member for the American Clinical Magnetoencephalography Society and the International Society for the Advancement of Clinical Magnetoencephalography.
Figures



References
-
- Uylings H.B., De Brabander J. Neuronal changes in normal human aging and Alzheimer's disease. Brain Cogn. 2002;49(3):268–276. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

