Marketing Authorization Applications Made to the European Medicines Agency in 2018-2019: What was the Contribution of Real-World Evidence?
- PMID: 34689339
- PMCID: PMC9299056
- DOI: 10.1002/cpt.2461
Marketing Authorization Applications Made to the European Medicines Agency in 2018-2019: What was the Contribution of Real-World Evidence?
Abstract
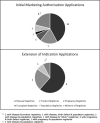
Information derived from routinely collected real-world data has for a long time been used to support regulatory decision making on the safety of drugs and has more recently been used to support marketing authorization submissions to regulators. There is a lack of detailed information on the use and types of this real-world evidence (RWE) as submitted to regulators. We used resources held by the European Medicines Agency (EMA) to describe the characteristics of RWE included in new marketing authorization applications (MAAs) and extensions of indication (EOIs) for already authorized products submitted to the EMA in 2018 and 2019. For MAAs, 63 of 158 products (39.9%) contained RWE with a total of 117 studies. For 31.7% of these products, the RWE submitted was derived from data collected before the planned authorization. The most common data sources were registries (60.3%) followed by hospital data (31.7%). RWE was mainly included to support safety (87.3%) and efficacy (49.2%) with cohort studies being the most frequently used study design (88.9%). For EOIs, 28 of 153 products (18.3%) contained RWE with a total of 36 studies. For 57.1% of these products, studies were conducted prior to the EOIs. RWE sources were mainly registries (35.6%) and hospital data (27.0%). RWE was typically used to support safety (82.1%) and efficacy (53.6%). Cohort studies were the most commonly used study design (87.6%). We conclude that there is widespread use of RWE to support evaluation of MAAs and EOIs submitted to the EMA and identify areas where further research is required.
© 2021 The Authors. Clinical Pharmacology & Therapeutics published by Wiley Periodicals LLC on behalf of American Society for Clinical Pharmacology and Therapeutics.
Conflict of interest statement
The authors declared no competing interests for this work.
Figures
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Research Materials