The value of the basophil activation test in the evaluation of patients reporting allergic reactions to the BNT162b2 mRNA COVID-19 vaccine
- PMID: 34689351
- PMCID: PMC8653141
- DOI: 10.1111/all.15148
The value of the basophil activation test in the evaluation of patients reporting allergic reactions to the BNT162b2 mRNA COVID-19 vaccine
Abstract
Background: mRNA-based COVID-19 vaccines have been reported to induce hypersensitivity reactions (HSR) in a small number of individuals. We aimed to evaluate the real-world incidence of the BNT162b2 mRNA COVID-19 vaccine HSR and to determine the value of the basophil activation test (BAT) in the allergological workup of patients reporting these reactions.
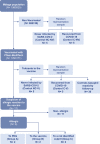
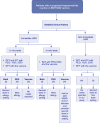
Methods: We prospectively enrolled patients with a clinical history indicative of HSR to the BNT162b2 mRNA COVID-19 vaccine. The allergological workup included skin testing (STs) and BAT with polyethylene glycol (PEG) and the vaccine. In those with negative allergy assessments, the administration of the second dose of the BNT162b2 mRNA COVID-19 vaccine was offered.
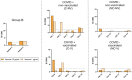
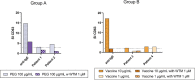
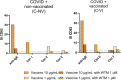
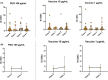
Results: Seventeen adults were included. Eleven cases (64.7%) tested negative in the allergological workup and tolerated the re-administration of the second dose of the vaccine and considered non-allergic. Six cases (35.3%) were considered allergic and classified into three groups: 2 subjects displayed positive STs and/or BAT to PEG (Group A), two individuals displayed positive BAT to the vaccine (Group B), and in 2 patients with moderate or severe reactions, the culprit was not identified, tested negative to STs and BAT to both PEG and vaccine (Group C). We further evaluated the value of BAT when the results were positive to the vaccine and negative to PEG by performing BAT in controls groups, finding positive BAT results in 50% of controls, all of them recovered from COVID-19 infection. In contrast, BAT was negative in patients who had not suffered from COVID-19 disease.
Conclusions: BAT can be used as a potential diagnostic tool for confirming allergy to PEG excipient but not to the vaccine as a positive result in BAT may indicate a past COVID-19 infection instead of an allergy.
Keywords: BNT162b2; COVID-19; allergic reactions; basophil activation test; vaccines.
© 20221 The Authors. Allergy published by European Academy of Allergy and Clinical Immunology and John Wiley & Sons Ltd.
Conflict of interest statement
No author has any conflicts of interest to disclose.
Figures
Comment in
-
Reply to correspondence: Basophil reactivity to BNT162b2 in COVID-19 convalescence.Allergy. 2022 Jul;77(7):2266-2267. doi: 10.1111/all.15208. Allergy. 2022. PMID: 35770813 Free PMC article. No abstract available.
-
Basophil reactivity to BNT162b2 in COVID-19 convalescence.Allergy. 2022 Dec;77(12):3704-3705. doi: 10.1111/all.15472. Allergy. 2022. PMID: 36441594 Free PMC article. No abstract available.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

