Muscle-generated BDNF (brain derived neurotrophic factor) maintains mitochondrial quality control in female mice
- PMID: 34689722
- PMCID: PMC9225428
- DOI: 10.1080/15548627.2021.1985257
Muscle-generated BDNF (brain derived neurotrophic factor) maintains mitochondrial quality control in female mice
Abstract
Mitochondrial remodeling is dysregulated in metabolic diseases but the underlying mechanism is not fully understood. We report here that BDNF (brain derived neurotrophic factor) provokes mitochondrial fission and clearance in skeletal muscle via the PRKAA/AMPK-PINK1-PRKN/Parkin and PRKAA-DNM1L/DRP1-MFF pathways. Depleting Bdnf expression in myotubes reduced fatty acid-induced mitofission and mitophagy, which was associated with mitochondrial elongation and impaired lipid handling. Muscle-specific bdnf knockout (MBKO) mice displayed defective mitofission and mitophagy, and accumulation of dysfunctional mitochondria in the muscle when they were fed with a high-fat diet (HFD). These animals also have exacerbated body weight gain, increased intramyocellular lipid deposition, reduced energy expenditure, poor metabolic flexibility, and more insulin resistance. In contrast, consuming a BDNF mimetic (7,8-dihydroxyflavone) increased mitochondrial content, and enhanced mitofission and mitophagy in the skeletal muscles. Hence, BDNF is an essential myokine to maintain mitochondrial quality and function, and its repression in obesity might contribute to impaired metabolism.Abbreviation: 7,8-DHF: 7,8-dihydroxyflavone; ACACA/ACC: acetyl Coenzyme A carboxylase alpha; ACAD: acyl-Coenzyme A dehydrogenase family; ACADVL: acyl-Coenzyme A dehydrogenase, very long chain; ACOT: acyl-CoA thioesterase; CAMKK2: calcium/calmodulin-dependent protein kinase kinase 2, beta; BDNF: brain derived neurotrophic factor; BNIP3: BCL2/adenovirus E1B interacting protein 3; BNIP3L/NIX: BCL2/adenovirus E1B interacting protein 3-like; CCL2/MCP-1: chemokine (C-C motif) ligand 2; CCL5: chemokine (C-C motif) ligand 5; CNS: central nervous system; CPT1B: carnitine palmitoyltransferase 1b, muscle; Cpt2: carnitine palmitoyltransferase 2; CREB: cAMP responsive element binding protein; DNM1L/DRP1: dynamin 1-like; E2: estrogen; EHHADH: enoyl-CoenzymeA hydratase/3-hydroxyacyl CoenzymeA dehydrogenase; ESR1/ER-alpha: estrogen receptor 1 (alpha); FA: fatty acid; FAO: fatty acid oxidation; FCCP: carbonyl cyanide-4-(trifluoromethoxy)phenylhydrazone; FFA: free fatty acids; FGF21: fibroblast growth factor 21; FUNDC1: FUN14 domain containing 1; HADHA: hydroxyacyl-CoA dehydrogenase trifunctional multienzyme complex subunit alpha; HFD: high-fat diet; iWAT: inguinal white adipose tissues; MAP1LC3A/LC3A: microtubule-associated protein 1 light chain 3 alpha; MBKO; muscle-specific bdnf knockout; IL6/IL-6: interleukin 6; MCEE: methylmalonyl CoA epimerase; MFF: mitochondrial fission factor; NTRK2/TRKB: neurotrophic tyrosine kinase, receptor, type 2; OPTN: optineurin; PA: palmitic acid; PARL: presenilin associated, rhomboid-like; PDH: pyruvate dehydrogenase; PINK1: PTEN induced putative kinase 1; PPARGC1A/PGC-1α: peroxisome proliferative activated receptor, gamma, coactivator 1 alpha; PRKAA/AMPK: protein kinase, AMP-activated, alpha 2 catalytic subunit; ROS: reactive oxygen species; TBK1: TANK-binding kinase 1; TG: triacylglycerides; TNF/TNFα: tumor necrosis factor; TOMM20: translocase of outer mitochondrial membrane 20; ULK1: unc-51 like kinase 1.
Keywords: BDNF; mitochondria; mitophagy; muscle; obesity.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
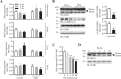

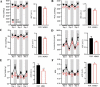

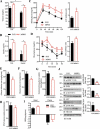

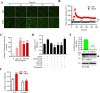

References
-
- Palikaras K, Lionaki E, Tavernarakis N. Mechanisms of mitophagy in cellular homeostasis, physiology and pathology. Nat Cell Biol. 2018;20(9):1013–1022. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
