Using Biomarkers to Predict Memantine Effects in Alzheimer's Disease: A Proposal and Proof-Of-Concept Demonstration
- PMID: 34690144
- PMCID: PMC8881988
- DOI: 10.3233/JAD-215029
Using Biomarkers to Predict Memantine Effects in Alzheimer's Disease: A Proposal and Proof-Of-Concept Demonstration
Abstract
Memantine's benefits in Alzheimer's disease (AD) are modest and heterogeneous. We tested the feasibility of using sensitivity to acute memantine challenge to predict an individual's clinical response. Eight participants completed a double-blind challenge study of memantine (placebo versus 20 mg) effects on autonomic, subjective, cognitive, and neurophysiological measures, followed by a 24-week unblinded active-dose therapeutic trial (10 mg bid). Study participation was well tolerated. Subgroups based on memantine sensitivity on specific laboratory measures differed in their clinical response to memantine, some by large effect sizes. It appears feasible to use biomarkers to predict clinical sensitivity to memantine.
Keywords: Alzheimer’s disease; event-related potentials; memantine; neurocognition; prepulse inhibition.
Figures

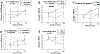
References
-
- Prince M, Wimo A, Guerchet M, Ali G-C, Wu Y-T, Prina M (2015) World Alzheimer Report 2015. The Global Impact of Dementia: An analysis of prevalence, incidence, cost and trends. Alzheimer’s Disease International, London, pp. 10–29.
-
- Areosa SA, Sherriff F, McShane R (2005) Memantine for dementia. Cochrane Database Syst Rev (4), CD 003154.pub3. - PubMed
-
- Bakchine S, Loft H (2008) Memantine treatment in patients with mild to moderate Alzheimer’s disease: Results of a randomized, double-blind, placebo-controlled 6-month study. J Alzheimers Dis 13, 97–107. - PubMed
-
- Peskind ER, Potkin SG, Pomara N, Ott BR, Graham SM, Olin JT, McDonald S (2006) Memantine treatment in mild to moderate Alzheimer disease: A 24-week randomized, controlled trial. Am J Geriatr Psychiatry 14, 704–715. - PubMed
-
- Pomara N, Ott BR, Peskind E, Resnick EM (2007) Memantine treatment of cognitive symptoms in mild to moderate Alzheimer disease: Secondary analyses from a placebo-controlled randomized trial. Alzheimer Dis Assoc Disord 21, 60–64. - PubMed

