Cooperativity in Transition Metal Tetrylene Complexes
- PMID: 34690540
- PMCID: PMC8518731
- DOI: 10.1002/ejic.202100460
Cooperativity in Transition Metal Tetrylene Complexes
Abstract
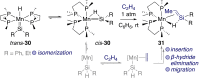
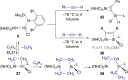
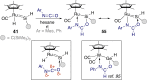
Cooperative reactivity between transition metals and ligands, or between two metals, has created significant opportunities for the development of new transformations that would be difficult to carry out with a single metal. Here we explore cooperativity between transition metals and divalent heavier group 14 elements (tetrylenes), a less-explored facet of the field of cooperativity. Tetrylenes combine their strong σ-donor properties with an empty p-orbital that can accept electron density. This ambiphilicity has allowed them to form metal tetrylene and metallotetrylene complexes that place a reactive site adjacent to the metal. We have selected examples to demonstrate what has been achieved so far regarding cooperative reactivity, as this already spans metal-, tetrylene- or multi-site-centred bond cleavage, cycloaddition, migration, metathesis, and insertion. We also highlight some challenges that need to be overcome for this cooperativity to make it to catalysis.
Keywords: Cooperative effects; Group 14 elements; Main group elements; Multi-site activation; Tetrylenes.
© 2021 The Authors. European Journal of Inorganic Chemistry published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




















References
-
- R. J. Lundgren, M. Stradiotto, Key Concepts in Ligand Design, Wiley: West Sussex, England, 2016.
-
- Prier C. K., MacMillan D. W. C., in Visible Light Photocatal. Org. Chem. (Eds.: Stephenson C. R. J., Yoon T. P., MacMillan D. W. C.), Wiley: Hoboken, New Jersey, 2018, Chapter 10, pp. 299–333.
Publication types
LinkOut - more resources
Full Text Sources
