Peptide Affinity Chromatography Applied to Therapeutic Antibodies Purification
- PMID: 34690622
- PMCID: PMC8525457
- DOI: 10.1007/s10989-021-10299-5
Peptide Affinity Chromatography Applied to Therapeutic Antibodies Purification
Abstract
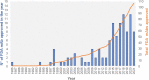
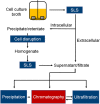
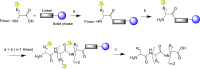
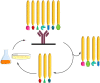
The interest in therapeutic monoclonal antibodies (mAbs) has significantly grown in the pharmaceutical industry, exceeding 100 FDA mAbs approved. Although the upstream processing of their industrial production has been significantly improved in the last years, the downstream processing still depends on immobilized protein A affinity chromatography. The high cost, low capacity and short half-life of immobilized protein A chromatography matrices, encouraged the design of alternative short-peptide ligands for mAb purification. Most of these peptides have been obtained by screening combinatorial peptide libraries. These low-cost ligands can be easily produced by solid-phase peptide synthesis and can be immobilized on chromatographic supports, thus obtaining matrices with high capacity and selectivity. Furthermore, matrices with immobilized peptide ligands have longer half-life than those with protein A due to the higher stability of the peptides. In this review the design and synthesis of peptide ligands, their immobilization on chromatographic supports and the evaluation of the affinity supports for their application in mAb purification is described.
Keywords: Biopharmaceuticals; Mass spectrometry; Monoclonal antibodies; Solid-phase peptide synthesis.
© The Author(s), under exclusive licence to Springer Nature B.V. 2021.
Conflict of interest statement
Conflict of interestNot applicable.
Figures
Similar articles
-
Design of Affinity Chromatography Peptide Ligands Through Combinatorial Peptide Library Screening.Methods Mol Biol. 2021;2178:217-243. doi: 10.1007/978-1-0716-0775-6_16. Methods Mol Biol. 2021. PMID: 33128753
-
Protocol for bevacizumab purification using Ac-PHQGQHIGVSK-agarose.MethodsX. 2019 Dec 16;7:100769. doi: 10.1016/j.mex.2019.12.010. eCollection 2020. MethodsX. 2019. PMID: 32021822 Free PMC article.
-
A short peptide fragment of the vascular endothelial growth factor as a novel ligand for bevacizumab purification.Protein Expr Purif. 2020 Jan;165:105500. doi: 10.1016/j.pep.2019.105500. Epub 2019 Sep 19. Protein Expr Purif. 2020. PMID: 31542564
-
Review on biomimetic affinity chromatography with short peptide ligands and its application to protein purification.J Chromatogr A. 2018 Oct 12;1571:1-15. doi: 10.1016/j.chroma.2018.07.082. Epub 2018 Jul 30. J Chromatogr A. 2018. PMID: 30097342 Review.
-
Antibody-like peptides as a novel purification tool for drugs design.Curr Pharm Des. 2006;12(2):191-203. doi: 10.2174/138161206775193082. Curr Pharm Des. 2006. PMID: 16454736 Review.
Cited by
-
[Preparation of a block copolymer-based temperature-responsive affinity chromatography stationary phase for antibody separation and purification].Se Pu. 2023 Dec;41(12):1045-1051. doi: 10.3724/SP.J.1123.2023.09028. Se Pu. 2023. PMID: 38093534 Free PMC article. Chinese.
-
Compendium on Food Crop Plants as a Platform for Pharmaceutical Protein Production.Int J Mol Sci. 2022 Mar 17;23(6):3236. doi: 10.3390/ijms23063236. Int J Mol Sci. 2022. PMID: 35328657 Free PMC article. Review.
References
-
- Barredo GR, Giudicessi SL, Martínez Ceron MC, Saavedra SL, Rodriguez S, Filgueira Risso L, Erra-Balsells R, Mahler G, Albericio F, Cascone O, Camperi SA. A short peptide fragment of the vascular endothelial growth factor as a novel ligand for bevacizumab purification. Protein Expr Purif. 2020;165:105500. doi: 10.1016/j.pep.2019.105500. - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
