Co-administration of vitamin D3 and Lacticaseibacillus paracasei DG increase 25-hydroxyvitamin D serum levels in mice
- PMID: 34690623
- PMCID: PMC8522538
- DOI: 10.1186/s13213-021-01655-3
Co-administration of vitamin D3 and Lacticaseibacillus paracasei DG increase 25-hydroxyvitamin D serum levels in mice
Abstract
Purpose: Subclinical vitamin D (vitD) deficiency enhances the predisposition to a myriad of acute and chronic pathologies in many people worldwide. Due to the scarcity of vitD-rich foods, the consumption of supplements or fortified foods can be required to maintain healthy serum levels of 25-hydroxyvitamin D [25(OH)D], and the major circulating form of vitD that is commonly measured in serum to determine the vitD status. Since the vitD absorption seems to resemble that of lipids, improved emulsification in the gut could favor vitD permeation through the enterocyte membrane. Contextually, we hypothesized that a microorganism with cholecalciferol (vitD3)-solubilization properties may potentially result in enhanced serum vitD levels.
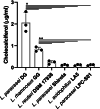
Methods and results: Six probiotic strains were screened for their ability to create a stable suspension of vitD3 in water: Lacticaseibacillus paracasei DG, L. paracasei LPC-S01, L. paracasei Shirota, L. rhamnosus GG, Limosilactobacillus reuteri DSM 17938, and Lactobacillus acidophilus LA5. The DG strain displayed the strongest vitD3 solubilization ability and, consequently, were used in an in vivo trial where a commercial preparation of vitD3 in refined olive oil was administered by gavage to CD-1 mice with or without the concurrent administration of L. paracasei DG. ELISA measurements showed that the DG strain significantly increased the serum levels of 25(OH) D when administered once a day for 1 week in association with the vitD3 supplement.
Conclusion: This preliminary pre-clinical study suggests that the combined administration of L. paracasei DG with an oil-based cholecalciferol supplement could contribute to the maintenance of the adequate 25(OH) D serum levels in people at risk of vitD deficiency.
Keywords: Bioavailability; Biosurfactant; Cholecalciferol supplementation; Probiotics; Vitamin D.
© The Author(s) 2021.
Conflict of interest statement
Competing interestsSofar S.p.A. is a private company that commercializes probiotic supplements containing L. paracasei DG. Walter Fiore and Valerio De Vitis are employees of Sofar S.p.A. Simone Guglielmetti is consultant of Sofar S.p.A. Other authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures


Similar articles
-
Highly selective whole-cell 25-hydroxyvitamin D3 synthesis using molybdenum-dependent C25-steroid dehydrogenase and cyclodextrin recycling.Microb Cell Fact. 2024 Jan 20;23(1):30. doi: 10.1186/s12934-024-02303-6. Microb Cell Fact. 2024. PMID: 38245746 Free PMC article.
-
The development of thyroid autoimmunity is potentially associated with the deficiency of vitamin D3 rather than vitamin D2 in euthyroid men.Thyroid Res. 2025 Mar 18;18(1):10. doi: 10.1186/s13044-025-00226-x. Thyroid Res. 2025. PMID: 40098187 Free PMC article.
-
Vitamin D deficiency is prevalent among idiopathic stone formers, but does correction pose any risk?Urolithiasis. 2017 Dec;45(6):535-543. doi: 10.1007/s00240-016-0954-x. Epub 2016 Dec 16. Urolithiasis. 2017. PMID: 27981376 Free PMC article.
-
Vitamin D Deficiency and Rheumatoid Arthritis.Clin Rev Allergy Immunol. 2017 Jun;52(3):373-388. doi: 10.1007/s12016-016-8577-0. Clin Rev Allergy Immunol. 2017. PMID: 27484684 Review.
-
25(OH)D3-enriched or fortified foods are more efficient at tackling inadequate vitamin D status than vitamin D3.Proc Nutr Soc. 2018 Aug;77(3):282-291. doi: 10.1017/S0029665117004062. Epub 2017 Nov 27. Proc Nutr Soc. 2018. PMID: 29173203 Free PMC article. Review.
Cited by
-
Lactobacillus rhamnosus GG Promotes Intestinal Vitamin D Absorption by Upregulating Vitamin D Transporters in Senile Osteoporosis.Calcif Tissue Int. 2022 Aug;111(2):162-170. doi: 10.1007/s00223-022-00975-z. Epub 2022 May 26. Calcif Tissue Int. 2022. PMID: 35616697
-
Vitamin D Status in Children with Autism Spectrum Disorders: Determinants and Effects of the Response to Probiotic Supplementation.Metabolites. 2022 Jul 1;12(7):611. doi: 10.3390/metabo12070611. Metabolites. 2022. PMID: 35888736 Free PMC article.
-
Vitamins as regulators of calcium-containing kidney stones - new perspectives on the role of the gut microbiome.Nat Rev Urol. 2023 Oct;20(10):615-637. doi: 10.1038/s41585-023-00768-5. Epub 2023 May 9. Nat Rev Urol. 2023. PMID: 37161031 Free PMC article. Review.
-
Tumorigenesis in Inflammatory Bowel Disease: Microbiota-Environment Interconnections.Cancers (Basel). 2023 Jun 15;15(12):3200. doi: 10.3390/cancers15123200. Cancers (Basel). 2023. PMID: 37370812 Free PMC article. Review.
-
Effects of Probiotics and Vitamin D3 Supplementation on Sports Performance Markers in Male Mixed Martial Arts Athletes: A Randomized Trial.Sports Med Open. 2023 May 16;9(1):31. doi: 10.1186/s40798-023-00576-6. Sports Med Open. 2023. PMID: 37193828 Free PMC article.
References
-
- Bogovic Matijasic B, Obermajer T, Lipoglavsek L, Sernel T, Locatelli I, Kos M, et al. Effects of synbiotic fermented milk containing Lactobacillus acidophilus La-5 and Bifidobacterium animalis ssp. lactis BB-12 on the fecal microbiota of adults with irritable bowel syndrome: a randomized double-blind, placebo-controlled trial. J Dairy Sci. 2016;99:5008–5021. doi: 10.3168/jds.2015-10743. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
