Efficacy and Safety of Rifaximin Versus Placebo or Other Active Drugs in Critical ill Patients With Hepatic Encephalopathy
- PMID: 34690751
- PMCID: PMC8533823
- DOI: 10.3389/fphar.2021.696065
Efficacy and Safety of Rifaximin Versus Placebo or Other Active Drugs in Critical ill Patients With Hepatic Encephalopathy
Abstract
Objective: Rifaximin has been approved for use as a first-line therapy for secondary prophylaxis of hepatic encephalopathy (HE). This article is to update existing evidence on efficacy and safety of rifaximin treatment and prevention for HE. Methods: We systematically searched multiple databases until January 31 2021. The studies compared rifaximin vs. placebo or other active drugs (i.e., nonabsorbable disaccharides, other antibiotics, L-ornithine-L-aspartate (LOLA), and probiotics) for patients with overt HE (OHE), minimal HE (MHE), and recurrent HE. Results: Twenty-eight randomized controlled trials with a total of 2979 patients were included. Compared with the controls, rifaximin significantly reduced HE grade (OHE: RR = 1.11, 95% CI = 1.02-1.21), improved the cognitive impairments (MHE: RR = 1.82, 95% CI = 1.12-2.93) and prevented the risk of HE recurrent episodes (RR = 1.33, 95% CI = 1.18-1.49). No statistical difference was observed in mortality between rifaximin and their controls (RR = 0.82, 95% CI = 0.54-1.24). The incidence of total adverse events in rifaximin-treated groups was significantly lower than that in the controls during the treatment period (RR = 0.73, 95% CI = 0.54-0.98). In addition, rifaximin treatment was better than other active drugs in improving psychometric indicators (mental state, flapping tremor and portosystemic encephalopathy (PSE) index) and reducing the risk of rehospitalization in HE patients. Conclusion: Rifaximin therapy is effective and well-tolerated in different types of HE, which might be recommended as an alternative to conventional oral drugs in clinical settings.
Keywords: efficacy; hepatic encephalopathy; meta-analysis; rifaximin; safety.
Copyright © 2021 Han, Luo, Wang, Zheng, Li, Mei and Wang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
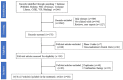
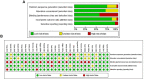
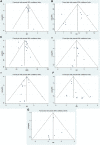



References
-
- American Association for the Study of Liver D. (2014). Hepatic Encephalopathy in Chronic Liver Disease: 2014 Practice Guideline by the European Association for the Study of the Liver and the American Association for the Study of Liver Diseases. J. Hepatol. 61 (3), 642–659. 10.1016/j.jhep.2014.05.042 - DOI - PubMed
-
- Aqeel A. R., Butt B., Naveed H., Arshad A. (2018). A Randomized Triple Blind, Placebo-Controlled Trial to Determine Effectiveness of Rifaximin in Preventing Hepatic Encephalopathy in Patients with Chronic Liver Disease. Indo Am. J. Pharm. Sci. 5 (4), 2728–2732.
Publication types
LinkOut - more resources
Full Text Sources
Medical

