Trimetazidine Modulates Mitochondrial Redox Status and Disrupted Glutamate Homeostasis in a Rat Model of Epilepsy
- PMID: 34690772
- PMCID: PMC8531497
- DOI: 10.3389/fphar.2021.735165
Trimetazidine Modulates Mitochondrial Redox Status and Disrupted Glutamate Homeostasis in a Rat Model of Epilepsy
Abstract
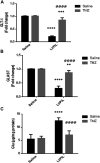
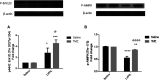
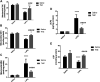
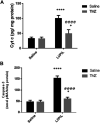
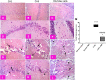
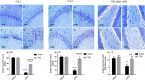
Mitochondrial oxidative status exerts an important role in modulating glia-neuron interplay during epileptogenesis. Trimetazidine (TMZ), a well-known anti-ischemic drug, has shown promising potential against a wide range of neurodegenerative disorders including epilepsy. Nevertheless, the exact mechanistic rationale behind its anti-seizure potential has not been fully elucidated yet. Herein, the impact of TMZ against mitochondrial oxidative damage as well as glutamate homeostasis disruption in the hippocampus has been investigated in rats with lithium/pilocarpine (Li/PIL) seizures. Animals received 3 mEq/kg i.p. LiCl3 followed by PIL (single i.p.; 150 mg/kg) 20 h later for induction of seizures with or without TMZ pretreatment (25 mg/kg; i.p.) for five consecutive days. Seizure score and seizure latency were observed. Mitochondrial redox status as well as ATP and uncoupling protein 2 was recorded. Moreover, glutamate homeostasis was unveiled. The present findings demonstrate the TMZ-attenuated Li/PIL seizure score and latency. It improved mitochondrial redox status, preserved energy production mechanisms, and decreased reactive astrocytes evidenced as decreased glial fibrillary acidic protein immune-stained areas in hippocampal tissue. In addition, it modulated phosphorylated extracellular signal-regulated kinases (p-ERK1/2) and p-AMP-activated protein kinase (p-AMPK) signaling pathways to reflect a verified anti-apoptotic effect. Consequently, it upregulated mRNA expression of astroglial glutamate transporters and reduced the elevated glutamate level. The current study demonstrates that TMZ exhibits robust anti-seizure and neuroprotective potentials. These effects are associated with its ability to modulate mitochondrial redox status, boost p-ERK1/2 and p-AMPK signaling pathways, and restore glutamate homeostasis in hippocampus.
Keywords: ERK1/2; astrogliosis; glutamate transporters; mitochondrial oxidative stress; trimetazidine.
Copyright © 2021 Al-Shorbagy, Wadie and El-Tanbouly.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
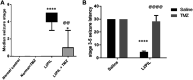

Similar articles
-
The protective effect of trimetazidine on myocardial ischemia/reperfusion injury through activating AMPK and ERK signaling pathway.Metabolism. 2016 Mar;65(3):122-30. doi: 10.1016/j.metabol.2015.10.022. Epub 2015 Oct 19. Metabolism. 2016. PMID: 26892523 Free PMC article.
-
Persistent impairment of mitochondrial and tissue redox status during lithium-pilocarpine-induced epileptogenesis.J Neurochem. 2010 Dec;115(5):1172-82. doi: 10.1111/j.1471-4159.2010.07013.x. Epub 2010 Oct 26. J Neurochem. 2010. PMID: 21219330 Free PMC article.
-
Effects of dexamethasone on the Li-pilocarpine model of epilepsy: protection against hippocampal inflammation and astrogliosis.J Neuroinflammation. 2018 Mar 5;15(1):68. doi: 10.1186/s12974-018-1109-5. J Neuroinflammation. 2018. PMID: 29506554 Free PMC article.
-
Sirtuin 1 in rat orthotopic liver transplantation: an IGL-1 preservation solution approach.World J Gastroenterol. 2015 Feb 14;21(6):1765-74. doi: 10.3748/wjg.v21.i6.1765. World J Gastroenterol. 2015. PMID: 25684941 Free PMC article.
-
Glutamate receptor antibodies in neurological diseases: anti-AMPA-GluR3 antibodies, anti-NMDA-NR1 antibodies, anti-NMDA-NR2A/B antibodies, anti-mGluR1 antibodies or anti-mGluR5 antibodies are present in subpopulations of patients with either: epilepsy, encephalitis, cerebellar ataxia, systemic lupus erythematosus (SLE) and neuropsychiatric SLE, Sjogren's syndrome, schizophrenia, mania or stroke. These autoimmune anti-glutamate receptor antibodies can bind neurons in few brain regions, activate glutamate receptors, decrease glutamate receptor's expression, impair glutamate-induced signaling and function, activate blood brain barrier endothelial cells, kill neurons, damage the brain, induce behavioral/psychiatric/cognitive abnormalities and ataxia in animal models, and can be removed or silenced in some patients by immunotherapy.J Neural Transm (Vienna). 2014 Aug;121(8):1029-75. doi: 10.1007/s00702-014-1193-3. Epub 2014 Aug 1. J Neural Transm (Vienna). 2014. PMID: 25081016 Review.
Cited by
-
Astrocyte mitochondria: Potential therapeutic targets for epilepsy.Heliyon. 2024 Apr 27;10(9):e29950. doi: 10.1016/j.heliyon.2024.e29950. eCollection 2024 May 15. Heliyon. 2024. PMID: 38756598 Free PMC article. Review.
-
Neuroprotective Effects of Trimetazidine against Cisplatin-Induced Peripheral Neuropathy: Involvement of AMPK-Mediated PI3K/mTOR, Nrf2, and NF-κB Signaling Axes.Oxid Med Cell Longev. 2024 Aug 20;2024:6612009. doi: 10.1155/2024/6612009. eCollection 2024. Oxid Med Cell Longev. 2024. PMID: 39502494 Free PMC article.
-
Mitochondrial dysfunction in epilepsy: mechanistic insights and clinical strategies.Mol Biol Rep. 2025 May 20;52(1):470. doi: 10.1007/s11033-025-10577-1. Mol Biol Rep. 2025. PMID: 40392243 Review.
-
Anticonvulsant effect of glycitin in pentylenetetrazol induced male Wistar rat model by targeting oxidative stress and Nrf2/HO-1 signaling.Front Pharmacol. 2024 Aug 23;15:1392325. doi: 10.3389/fphar.2024.1392325. eCollection 2024. Front Pharmacol. 2024. PMID: 39246658 Free PMC article.
-
Trimetazidine Improves Mitochondrial Dysfunction in SOD1G93A Cellular Models of Amyotrophic Lateral Sclerosis through Autophagy Activation.Int J Mol Sci. 2024 Mar 13;25(6):3251. doi: 10.3390/ijms25063251. Int J Mol Sci. 2024. PMID: 38542223 Free PMC article.
References
-
- Ahmed L. A., Shehata N. I., Abdelkader N. F., Khattab M. M. (2014). Tempol, a Superoxide Dismutase Mimetic Agent, Ameliorates Cisplatin-Induced Nephrotoxicity Through Alleviation of Mitochondrial Dysfunction in Mice. PLoS One. 9 (10), e108889. Erratum in: PLoS One. 2014;9(12):e115983. 10.1371/journal.pone.0108889 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

