The Lipocalin Apolipoprotein D Functional Portrait: A Systematic Review
- PMID: 34690812
- PMCID: PMC8530192
- DOI: 10.3389/fphys.2021.738991
The Lipocalin Apolipoprotein D Functional Portrait: A Systematic Review
Abstract
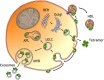
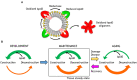
Apolipoprotein D is a chordate gene early originated in the Lipocalin protein family. Among other features, regulation of its expression in a wide variety of disease conditions in humans, as apparently unrelated as neurodegeneration or breast cancer, have called for attention on this gene. Also, its presence in different tissues, from blood to brain, and different subcellular locations, from HDL lipoparticles to the interior of lysosomes or the surface of extracellular vesicles, poses an interesting challenge in deciphering its physiological function: Is ApoD a moonlighting protein, serving different roles in different cellular compartments, tissues, or organisms? Or does it have a unique biochemical mechanism of action that accounts for such apparently diverse roles in different physiological situations? To answer these questions, we have performed a systematic review of all primary publications where ApoD properties have been investigated in chordates. We conclude that ApoD ligand binding in the Lipocalin pocket, combined with an antioxidant activity performed at the rim of the pocket are properties sufficient to explain ApoD association with different lipid-based structures, where its physiological function is better described as lipid-management than by long-range lipid-transport. Controlling the redox state of these lipid structures in particular subcellular locations or extracellular structures, ApoD is able to modulate an enormous array of apparently diverse processes in the organism, both in health and disease. The new picture emerging from these data should help to put the physiological role of ApoD in new contexts and to inspire well-focused future research.
Keywords: ApoD; extracellular vesicles; lipid peroxidation; lipoprotein particles; lysosome; membrane management; oxidative stress; protein physiology.
Copyright © 2021 Sanchez and Ganfornina.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Apolipoprotein D in Oxidative Stress and Inflammation.Antioxidants (Basel). 2023 Apr 28;12(5):1027. doi: 10.3390/antiox12051027. Antioxidants (Basel). 2023. PMID: 37237893 Free PMC article. Review.
-
Protecting cells by protecting their vulnerable lysosomes: Identification of a new mechanism for preserving lysosomal functional integrity upon oxidative stress.PLoS Genet. 2017 Feb 9;13(2):e1006603. doi: 10.1371/journal.pgen.1006603. eCollection 2017 Feb. PLoS Genet. 2017. PMID: 28182653 Free PMC article.
-
The Neuroprotective Lipocalin Apolipoprotein D Stably Interacts with Specific Subtypes of Detergent-Resistant Membrane Domains in a Basigin-Independent Manner.Mol Neurobiol. 2022 Jul;59(7):4015-4029. doi: 10.1007/s12035-022-02829-z. Epub 2022 Apr 22. Mol Neurobiol. 2022. PMID: 35460054 Free PMC article.
-
Apolipoprotein D-mediated preservation of lysosomal function promotes cell survival and delays motor impairment in Niemann-Pick type A disease.Neurobiol Dis. 2020 Oct;144:105046. doi: 10.1016/j.nbd.2020.105046. Epub 2020 Aug 13. Neurobiol Dis. 2020. PMID: 32798728
-
Apolipoprotein D takes center stage in the stress response of the aging and degenerative brain.Neurobiol Aging. 2014 Jul;35(7):1632-42. doi: 10.1016/j.neurobiolaging.2014.01.148. Epub 2014 Feb 5. Neurobiol Aging. 2014. PMID: 24612673 Free PMC article. Review.
Cited by
-
Promotion effect of TGF-β-Zfp423-ApoD pathway on lip sensory recovery after nerve sacrifice caused by nerve collateral compensation.Int J Oral Sci. 2023 Jun 8;15(1):23. doi: 10.1038/s41368-023-00230-7. Int J Oral Sci. 2023. PMID: 37286538 Free PMC article.
-
Apolipoprotein D in Oxidative Stress and Inflammation.Antioxidants (Basel). 2023 Apr 28;12(5):1027. doi: 10.3390/antiox12051027. Antioxidants (Basel). 2023. PMID: 37237893 Free PMC article. Review.
-
Dual role of Apolipoprotein D as long-term instructive factor and acute signal conditioning microglial secretory and phagocytic responses.Front Cell Neurosci. 2023 Jan 26;17:1112930. doi: 10.3389/fncel.2023.1112930. eCollection 2023. Front Cell Neurosci. 2023. PMID: 36779011 Free PMC article.
-
Favourable HDL composition in endurance athletes is not associated with changes in HDL in vitro antioxidant and endothelial anti-inflammatory function.Biosci Rep. 2024 Oct 30;44(10):BSR20241165. doi: 10.1042/BSR20241165. Biosci Rep. 2024. PMID: 39344511 Free PMC article.
-
Plasma apolipoprotein concentrations and occurrence of cardiovascular events in the general population: an exploratory analysis.Atheroscler Plus. 2025 May 8;60:35-42. doi: 10.1016/j.athplu.2025.04.003. eCollection 2025 Jun. Atheroscler Plus. 2025. PMID: 40485857 Free PMC article.
References
-
- Akiba S., Arai N., Kusuoku H., Takagi Y., Hagura T., Takeuchi K., et al. . (2011). The N-terminal amino acid of apolipoprotein D is putatively covalently bound to 3-hydroxy-3-methyl hexanoic acid, a key odour compound in axillary sweat. Int. J. Cosmet. Sci. 33, 283–286. 10.1111/j.1468-2494.2010.00636.x - DOI - PubMed
-
- Albers J. J., Taggart H. M., Applebaum-Bowden D., Haffner S., Chesnut C. H., Hazzard W. R. (1984). Reduction of lecithin-cholesterol acyltransferase, apolipoprotein D and the Lp(a) lipoprotein with the anabolic steroid stanozolol. Biochim. Biophys. Acta 795, 293–296. 10.1016/0005-2760(84)90078-X - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

