Gut Dysbiosis, Bacterial Colonization and Translocation, and Neonatal Sepsis in Very-Low-Birth-Weight Preterm Infants
- PMID: 34690993
- PMCID: PMC8529156
- DOI: 10.3389/fmicb.2021.746111
Gut Dysbiosis, Bacterial Colonization and Translocation, and Neonatal Sepsis in Very-Low-Birth-Weight Preterm Infants
Abstract
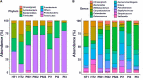
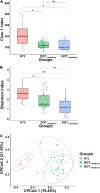
Gut dysbiosis may precede neonatal sepsis, but the association is still not well-understood. The goal of this study is to investigate the association between gut microbiota and neonatal sepsis, and to seek the evidence of colonization of pathogenic bacteria in the gut before evolving into an invasive infection. A prospective cohort study examined fecal microbiota composition in preterm infants with and without sepsis. Thirty-two very-low-birth-weight (VLBW) preterm infants and 10 healthy term infants as controls were enrolled. The fecal samples collected from the participants at the first, fourth, and seventh weeks of life underwent 16S rRNA amplicon sequencing for measurement of the diversity and composition of the microbiota. The bacterial isolates causing neonatal sepsis were genome sequenced. PCR was performed to confirm the translocation of the bacteria from the gut to the blood. The results showed that VLBW preterm infants with sepsis had lower microbial diversity in the gut at birth compared to preterm infants without sepsis and term infants. The composition of gut microbiome in preterm infants was similar to healthy terms at birth but evolved toward dysbiosis with increasing Proteobacteria and decreasing Firmicutes weeks later. The strain-specific PCR confirmed the presence of causative pathogens in the gut in 4 (40%) out of 10 VLBW preterms with sepsis before or at onset of sepsis, and persistence of the colonization for weeks after antibiotic treatment. The same bacterial strain could horizontally spread to cause infection in other infants. Prolonged antibiotic exposure significantly reduced beneficial Bifidobacterium and Lactobacillus in the gut. In conclusion, preterm infants with gut dysbiosis are at risk for neonatal sepsis, and the causative pathogens may be from the gut and persist to spread horizontally. The association of increased Proteobacteria abundance and decrease in microbiome diversity suggests the need for interventions targeting the gut microbiome to prevent dysbiosis and sepsis in VLBW preterm infants.
Keywords: dysbiosis; gut; microbiota; preterm infants; sepsis; very-low-birth-weight.
Copyright © 2021 Lee, Feng, Yeh, Lien, Chen, Zhou and Chiu.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



References
LinkOut - more resources
Full Text Sources
Medical

