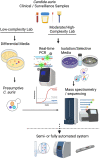
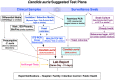
So Many Diagnostic Tests, So Little Time: Review and Preview of Candida auris Testing in Clinical and Public Health Laboratories
- PMID: 34691009
- PMCID: PMC8529189
- DOI: 10.3389/fmicb.2021.757835
So Many Diagnostic Tests, So Little Time: Review and Preview of Candida auris Testing in Clinical and Public Health Laboratories
Abstract
The recognition of a new yeast, Candida auris, in 2009 in East Asia, and its rapid global spread, was a reminder of the threats posed by multidrug-resistant fungal pathogens. C. auris had likely remained unrecognized for a long time as accurate tests were not available. The laboratory community responded to the C. auris challenge by publishing 35 new or revised diagnostic methods between 2014 and early 2021. The commercial sector also modified existing diagnostic devices. These C. auris diagnostic tests run the gamut from traditional culture-based differential and selective media, biochemical assimilations, and rapid protein profiles, as well as culture-independent DNA-based diagnostics. We provide an overview of these developments, especially the tests with validation data that were subsequently adopted for common use. We share a workflow developed in our laboratory to process over 37,000 C. auris surveillance samples and 5,000 C. auris isolates from the outbreak in the New York metropolitan area. Our preview covers new devices and diagnostic approaches on the horizon based on microfluidics, optics, and nanotechnology. Frontline laboratories need rapid, cheap, stable, and easy-to-implement tests to improve C. auris diagnosis, surveillance, patient isolation, admission screening, and environmental control. Among the urgent needs is a lateral flow assay or similar device for presumptive C. auris identification. All laboratories will benefit from devices that allow rapid antifungal susceptibility testing, including detection of mutations conferring drug resistance. Hopefully, multiplex test panels are on the horizon for synergy of C. auris testing with ongoing surveillance of other healthcare-associated infections. C. auris genome analysis has a proven role for outbreak investigations, and diagnostic laboratories need quick access to regional and national genome analysis networks.
Keywords: LAMP; MALDI-TOF MS; PCR; biosensor; clinical; healthcare-associated infections; laboratory-developed tests; real-time PCR; surveillance.
Copyright © 2021 Dennis, Chaturvedi and Chaturvedi.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Laboratory Analysis of an Outbreak of Candida auris in New York from 2016 to 2018: Impact and Lessons Learned.J Clin Microbiol. 2020 Mar 25;58(4):e01503-19. doi: 10.1128/JCM.01503-19. Print 2020 Mar 25. J Clin Microbiol. 2020. PMID: 31852764 Free PMC article.
-
Candida auris: Diagnostic Challenges and Emerging Opportunities for the Clinical Microbiology Laboratory.Curr Fungal Infect Rep. 2021;15(3):116-126. doi: 10.1007/s12281-021-00420-y. Epub 2021 Jun 23. Curr Fungal Infect Rep. 2021. PMID: 34178208 Free PMC article. Review.
-
Validation of a qualitative real-time PCR assay for the detection of Candida auris in hospital inpatient screening.J Clin Microbiol. 2024 Jun 12;62(6):e0015824. doi: 10.1128/jcm.00158-24. Epub 2024 May 1. J Clin Microbiol. 2024. PMID: 38690882 Free PMC article.
-
A Rapid and Automated Sample-to-Result Candida auris Real-Time PCR Assay for High-Throughput Testing of Surveillance Samples with the BD Max Open System.J Clin Microbiol. 2019 Sep 24;57(10):e00630-19. doi: 10.1128/JCM.00630-19. Print 2019 Oct. J Clin Microbiol. 2019. PMID: 31391229 Free PMC article.
-
Identification of Drug Resistant Candida auris.Front Microbiol. 2019 Aug 20;10:1918. doi: 10.3389/fmicb.2019.01918. eCollection 2019. Front Microbiol. 2019. PMID: 31481947 Free PMC article. Review.
Cited by
-
Assessment of LAMPAuris for Rapid Detection of Candida auris in Clinical Specimens.Mycopathologia. 2024 Sep 23;189(5):87. doi: 10.1007/s11046-024-00892-9. Mycopathologia. 2024. PMID: 39312077
-
Frequency of Detection of Candida auris Colonization Outside a Highly Endemic Setting: What Is the Optimal Strategy for Screening of Carriage?J Fungi (Basel). 2023 Dec 29;10(1):26. doi: 10.3390/jof10010026. J Fungi (Basel). 2023. PMID: 38248936 Free PMC article.
-
Candida auris in skilled nursing facilities.Ther Adv Infect Dis. 2023 Jul 29;10:20499361231189958. doi: 10.1177/20499361231189958. eCollection 2023 Jan-Dec. Ther Adv Infect Dis. 2023. PMID: 37529375 Free PMC article. Review.
-
Operational impact of decreased turnaround times for Candida auris screening tests in a tertiary academic medical center.Antimicrob Steward Healthc Epidemiol. 2023 Oct 18;3(1):e176. doi: 10.1017/ash.2023.445. eCollection 2023. Antimicrob Steward Healthc Epidemiol. 2023. PMID: 38028904 Free PMC article.
-
First imported case of Candida auris infection in Milan, Italy: genomic characterisation.Infection. 2024 Aug;52(4):1633-1638. doi: 10.1007/s15010-024-02232-x. Epub 2024 Apr 1. Infection. 2024. PMID: 38557967 Free PMC article.
References
Publication types
LinkOut - more resources
Full Text Sources