Targeting Microglial α-Synuclein/TLRs/NF-kappaB/NLRP3 Inflammasome Axis in Parkinson's Disease
- PMID: 34691027
- PMCID: PMC8531525
- DOI: 10.3389/fimmu.2021.719807
Targeting Microglial α-Synuclein/TLRs/NF-kappaB/NLRP3 Inflammasome Axis in Parkinson's Disease
Abstract
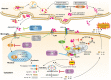
According to emerging studies, the excessive activation of microglia and the subsequent release of pro-inflammatory cytokines play important roles in the pathogenesis and progression of Parkinson's disease (PD). However, the exact mechanisms governing chronic neuroinflammation remain elusive. Findings demonstrate an elevated level of NLRP3 inflammasome in activated microglia in the substantia nigra of PD patients. Activated NLRP3 inflammasome aggravates the pathology and accelerates the progression of neurodegenerative diseases. Abnormal protein aggregation of α-synuclein (α-syn), a pathologically relevant protein of PD, were reported to activate the NLRP3 inflammasome of microglia through interaction with toll-like receptors (TLRs). This eventually releases pro-inflammatory cytokines through the translocation of nuclear factor kappa-B (NF-κB) and causes an impairment of mitochondria, thus damaging the dopaminergic neurons. Currently, therapeutic drugs for PD are primarily aimed at providing relief from its clinical symptoms, and there are no well-established strategies to halt or reverse this disease. In this review, we aimed to update existing knowledge on the role of the α-syn/TLRs/NF-κB/NLRP3 inflammasome axis and microglial activation in PD. In addition, this review summarizes recent progress on the α-syn/TLRs/NF-κB/NLRP3 inflammasome axis of microglia as a potential target for PD treatment by inhibiting microglial activation.
Keywords: NLRP3 inflammasome; Parkinson’s disease; microglia; neuroinflammation; α-synuclein.
Copyright © 2021 Li, Xia, Yin, Wan, Hu, Kou, Sun, Wu, Zhou, Huang, Xiong and Wang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
NF-κB/NLRP3 inflammasome axis and risk of Parkinson's disease in Type 2 diabetes mellitus: A narrative review and new perspective.J Cell Mol Med. 2023 Jul;27(13):1775-1789. doi: 10.1111/jcmm.17784. Epub 2023 May 21. J Cell Mol Med. 2023. PMID: 37210624 Free PMC article. Review.
-
Echinacoside exerts neuroprotection via suppressing microglial α-synuclein/TLR2/NF-κB/NLRP3 axis in parkinsonian models.Phytomedicine. 2024 Jan;123:155230. doi: 10.1016/j.phymed.2023.155230. Epub 2023 Nov 19. Phytomedicine. 2024. PMID: 38000105
-
Toll-like receptors and NLRP3 inflammasome-dependent pathways in Parkinson's disease: mechanisms and therapeutic implications.J Neurol. 2023 Mar;270(3):1346-1360. doi: 10.1007/s00415-022-11491-3. Epub 2022 Dec 3. J Neurol. 2023. PMID: 36460875 Free PMC article. Review.
-
The circadian clock protein Rev-erbα provides neuroprotection and attenuates neuroinflammation against Parkinson's disease via the microglial NLRP3 inflammasome.J Neuroinflammation. 2022 Jun 6;19(1):133. doi: 10.1186/s12974-022-02494-y. J Neuroinflammation. 2022. PMID: 35668454 Free PMC article.
-
Dl-3-n-Butylphthalide Rescues Dopaminergic Neurons in Parkinson's Disease Models by Inhibiting the NLRP3 Inflammasome and Ameliorating Mitochondrial Impairment.Front Immunol. 2021 Dec 1;12:794770. doi: 10.3389/fimmu.2021.794770. eCollection 2021. Front Immunol. 2021. PMID: 34925379 Free PMC article.
Cited by
-
Platelet Activating Factor Receptor Exaggerates Microglia-Mediated Microenvironment by IL10-STAT3 Signaling: A Novel Potential Biomarker and Target for Diagnosis and Treatment of Alzheimer's Disease.Front Aging Neurosci. 2022 Apr 28;14:856628. doi: 10.3389/fnagi.2022.856628. eCollection 2022. Front Aging Neurosci. 2022. PMID: 35572136 Free PMC article.
-
Punicalagin's Protective Effects on Parkinson's Progression in Socially Isolated and Socialized Rats: Insights into Multifaceted Pathway.Pharmaceutics. 2023 Oct 4;15(10):2420. doi: 10.3390/pharmaceutics15102420. Pharmaceutics. 2023. PMID: 37896179 Free PMC article.
-
Regulation of microglia phagocytosis and potential involvement of exercise.Front Cell Neurosci. 2022 Jul 25;16:953534. doi: 10.3389/fncel.2022.953534. eCollection 2022. Front Cell Neurosci. 2022. PMID: 35959472 Free PMC article. Review.
-
Gut Microbial Metabolome and Dysbiosis in Neurodegenerative Diseases: Psychobiotics and Fecal Microbiota Transplantation as a Therapeutic Approach-A Comprehensive Narrative Review.Int J Mol Sci. 2023 Aug 27;24(17):13294. doi: 10.3390/ijms241713294. Int J Mol Sci. 2023. PMID: 37686104 Free PMC article. Review.
-
Research trends of ferroptosis and pyroptosis in Parkinson's disease: a bibliometric analysis.Front Mol Neurosci. 2024 May 16;17:1400668. doi: 10.3389/fnmol.2024.1400668. eCollection 2024. Front Mol Neurosci. 2024. PMID: 38817551 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

