Advances in Universal CAR-T Cell Therapy
- PMID: 34691052
- PMCID: PMC8526896
- DOI: 10.3389/fimmu.2021.744823
Advances in Universal CAR-T Cell Therapy
Abstract
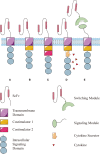
Chimeric antigen receptor T (CAR-T) cell therapy achieved extraordinary achievements results in antitumor treatments, especially against hematological malignancies, where it leads to remarkable, long-term antineoplastic effects with higher target specificity. Nevertheless, some limitations persist in autologous CAR-T cell therapy, such as high costs, long manufacturing periods, and restricted cell sources. The development of a universal CAR-T (UCAR-T) cell therapy is an attractive breakthrough point that may overcome most of these drawbacks. Here, we review the progress and challenges in CAR-T cell therapy, especially focusing on comprehensive comparison in UCAR-T cell therapy to original CAR-T cell therapy. Furthermore, we summarize the developments and concerns about the safety and efficiency of UCAR-T cell therapy. Finally, we address other immune cells, which might be promising candidates as a complement for UCAR-T cells. Through a detailed overview, we describe the current landscape and explore the prospect of UCAR-T cell therapy.
Keywords: CRISPR/Cas9; cellular immunotherapy; chimeric antigen receptor T cell therapy; gene editing; universal chimeric antigen receptor T cell therapy.
Copyright © 2021 Lin, Cheng, Mu, Zhou and Zhu.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

