Cell Wall Composition and Structure Define the Developmental Fate of Embryogenic Microspores in Brassica napus
- PMID: 34691114
- PMCID: PMC8526864
- DOI: 10.3389/fpls.2021.737139
Cell Wall Composition and Structure Define the Developmental Fate of Embryogenic Microspores in Brassica napus
Abstract
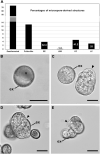
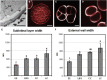
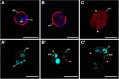
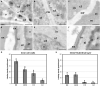
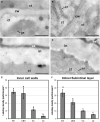
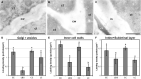
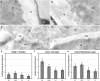
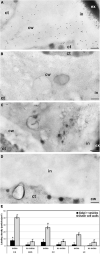
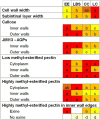
Microspore cultures generate a heterogeneous population of embryogenic structures that can be grouped into highly embryogenic structures [exine-enclosed (EE) and loose bicellular structures (LBS)] and barely embryogenic structures [compact callus (CC) and loose callus (LC) structures]. Little is known about the factors behind these different responses. In this study we performed a comparative analysis of the composition and architecture of the cell walls of each structure by confocal and quantitative electron microscopy. Each structure presented specific cell wall characteristics that defined their developmental fate. EE and LBS structures, which are responsible for most of the viable embryos, showed a specific profile with thin walls rich in arabinogalactan proteins (AGPs), highly and low methyl-esterified pectin and callose, and a callose-rich subintinal layer not necessarily thick, but with a remarkably high callose concentration. The different profiles of EE and LBS walls support the development as suspensorless and suspensor-bearing embryos, respectively. Conversely, less viable embryogenic structures (LC) presented the thickest walls and the lowest values for almost all of the studied cell wall components. These cell wall properties would be the less favorable for cell proliferation and embryo progression. High levels of highly methyl-esterified pectin are necessary for wall flexibility and growth of highly embryogenic structures. AGPs seem to play a role in cell wall stiffness, possibly due to their putative role as calcium capacitors, explaining the positive relationship between embryogenic potential and calcium levels.
Keywords: androgenesis; arabinogalactan proteins; callose; cell totipotency; cell wall; cellulose; microspore embryogenesis; subintinal layer.
Copyright © 2021 Camacho-Fernández, Seguí-Simarro, Mir, Boutilier and Corral-Martínez.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
-
- Abramova L. I., Avalkina N. A., Golubeva E. A., Pyzhenkova Z. S., Golubovskaya I. N. (2003). Synthesis and deposition of callose in anthers and ovules of meiotic mutants of maize (Zea mays). Russ. J. Plant Physiol. 50 324–329. 10.1023/a:1023866019102 - DOI
-
- Albersheim P., Darvill A., Roberts K., Sederoff R., Staehelin A. (2011). Plant Cell Walls. New York: Garland Science.
-
- Borderies G., Le Bechec M., Rossignol M., Lafitte C., Le Deunff E., Beckert M., et al. (2004). Characterization of proteins secreted during maize microspore culture: arabinogalactan proteins (AGPs) stimulate embryo development. Eur. J. Cell Biol. 83 205–212. - PubMed

