Tanshinone IIa Induces Autophagy and Apoptosis via PI3K/Akt/mTOR Axis in Acute Promyelocytic Leukemia NB4 Cells
- PMID: 34691211
- PMCID: PMC8536410
- DOI: 10.1155/2021/3372403
Tanshinone IIa Induces Autophagy and Apoptosis via PI3K/Akt/mTOR Axis in Acute Promyelocytic Leukemia NB4 Cells
Abstract
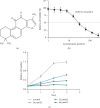
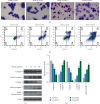
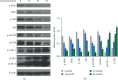
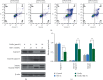
Tanshinone IIa (TanIIa), an ingredient of Radix Salviae Miltiorrhizae, has an anticancer effect on various solid tumors with high efficiency and low toxicity. Nonetheless, the underlying role of TanIIa in acute promyelocytic leukemia (APL) remains unclear. Here, we revealed that TanIIa drastically inhibited NB4 cell viability with an IC50 value of 31.25 μmol/L. Using flow cytometry apoptosis assay, we identified that TanIIa dose-dependently exacerbated NB4 cell apoptosis. Mechanistically, TanIIa upregulated apoptotic factor levels, namely, cleaved-caspase 9, cleaved-caspase 3, and cleaved-PARP-1. Moreover, we noticed that TanIIa dose-dependently suppressed the PI3K/Akt/mTOR axis. This axis not only functions as an essential antiapoptotic modulator but also serves as a suppressant regulator of autophagy. Correspondingly, we detected the levels of autophagic marker, namely, LC3B, which were increased after the TanIIa treatment. Furthermore, the autophagy inhibitor Baf-A1 could effectively reverse the TanIIa-induced apoptosis, manifesting that TanIIa eliminated NB4 cells in an autophagy-dependent manner. In conclusion, tanshinone IIa exerts anti-APL effects through triggering autophagy and apoptosis in NB4 cells.
Copyright © 2021 Yiming Pan et al.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
References
-
- Shigeno K., Naito K., Sahara N., et al. Arsenic trioxide therapy in relapsed or refractory Japanese patients with acute promyelocytic leukemia: updated outcomes of the phase II study and postremission therapies. International Journal of Hematology . 2005;82(3):224–229. doi: 10.1532/ijh97.05044. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

