Efficacy of Bifidobacterium Triple Viable Enteric-Coated Capsules Combined with Enteral Nutrition on Patients with Chronic Critical Illness and Influence on Immune and Coagulation Function
- PMID: 34691213
- PMCID: PMC8536439
- DOI: 10.1155/2021/3718255
Efficacy of Bifidobacterium Triple Viable Enteric-Coated Capsules Combined with Enteral Nutrition on Patients with Chronic Critical Illness and Influence on Immune and Coagulation Function
Retraction in
-
Retracted: Efficacy of Bifidobacterium Triple Viable Enteric-Coated Capsules Combined with Enteral Nutrition on Patients with Chronic Critical Illness and Influence on Immune and Coagulation Function.Evid Based Complement Alternat Med. 2023 Dec 13;2023:9835982. doi: 10.1155/2023/9835982. eCollection 2023. Evid Based Complement Alternat Med. 2023. PMID: 38125195 Free PMC article.
Abstract
Objective: To investigate the efficacy of enteric-coated Bifidobacterium triple viable capsules combined with enteral nutrition in the treatment of patients with chronic critical illness (CCI) and their effects on the immune and coagulation function of patients.
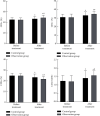
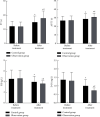
Methods: 106 CCI patients admitted to the intensive care unit of our hospital from December 2018 to March 2020 were selected as the research objects, and they were randomly divided into the control group (n = 53) and the observation group (n = 53). The control group was given symptomatic treatment, etiological treatment, clinical nursing, enteral nutrition support, and other conventional treatment methods according to the patient's condition. On this basis, the observation group was treated with enteric-coated Bifidobacterium triple viable capsules, and both groups were treated for 14 days. All patients were followed up for 3 months after treatment, and their death/cure prognosis was recorded. The acute physiological and chronic health (APACHE II) scoring system was used to evaluate the acute physiological and chronic health status of the two groups before and after treatment, and the organs of the patients were scored with sepsis-related organ failure assessment (SOFA) score. T lymphocyte subsets (CD3+, CD4+, CD8+, and CD4+/CD8+), prothrombin time (PT), activated partial thrombin time (APTT), fibrinogen (FIB), and D-dimer (DD) were measured before and after treatment.
Results: The cure rate of the observation group was slightly higher than that of the control group, and the mortality rate was slightly lower than that of the control group, but the difference was not statistically significant (P < 0.05). After treatment, the APACHE II and SOFA scores of the two groups were lower than before treatment, and the APACHE II and SOFA scores of the observation group were lower than those of the control group, and the differences were both statistically significant (P < 0.05). After treatment, the levels of CD3+, CD4+, and CD4+/CD8+ in the two groups were higher than those before treatment, and the levels of CD8+ were lower than before treatment. The CD3+, CD4+, and CD4+/CD8+ levels of the observation group were higher than those of the control group, and the CD8+ levels were lower than the control group, and the differences were both statistically significant (P < 0.05). After treatment, the PT and APTT levels of the two groups of patients were higher than those before treatment, and the levels of FIB and DD were lower than those before treatment. The PT and APTT levels of the observation group were higher than those of the control group, and the FIB and DD levels were lower than those of the control group, and the differences were both statistically significant (P < 0.05).
Conclusion: The combination of enteric-coated Bifidobacterium triple viable capsules and enteral nutrition for CCI has high cure rate, which can not only improve the patients' physiological health status and organ dysfunction but also effectively improve the patients' immune and coagulation function, which is worthy of promotion.
Copyright © 2021 Wei Wang et al.
Conflict of interest statement
The authors declare that they have no conflicts of interest.
Figures

Similar articles
-
Effect of Microecological Regulator Combined with Enteral Nutrition on Immune and Coagulation Function in Patients with Chronic Critical Illness.Cell Mol Biol (Noisy-le-grand). 2023 Feb 28;69(2):133-137. doi: 10.14715/cmb/2023.69.2.22. Cell Mol Biol (Noisy-le-grand). 2023. PMID: 37224032
-
[Study on the value of prothrombin time for predicting the severity and prognosis of septic patients].Zhonghua Wei Zhong Bing Ji Jiu Yi Xue. 2022 Jul;34(7):682-688. doi: 10.3760/cma.j.cn121430-20210614-00876. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue. 2022. PMID: 36100403 Chinese.
-
[Effect of self-made Qingyuan Shenghua decoction on coagulation dysfunction in patients with sepsis].Zhonghua Wei Zhong Bing Ji Jiu Yi Xue. 2021 Aug;33(8):944-948. doi: 10.3760/cma.j.cn121430-20201231-00790. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue. 2021. PMID: 34590561 Chinese.
-
Therapeutic effect of Bifidobacterium combined with early enteral nutrition in the treatment of severe acute pancreatitis: a pilot study.Eur Rev Med Pharmacol Sci. 2018 Jun;22(12):4018-4024. doi: 10.26355/eurrev_201806_15288. Eur Rev Med Pharmacol Sci. 2018. PMID: 29949178
-
[Prognostic value of coagulation function combined with acute physiology and chronic health evaluation II and sequential organ failure assessment scores for patients with bloodstream infection].Zhonghua Wei Zhong Bing Ji Jiu Yi Xue. 2021 Dec;33(12):1434-1439. doi: 10.3760/cma.j.cn121430-20210910-01361. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue. 2021. PMID: 35131009 Chinese.
Cited by
-
Retracted: Efficacy of Bifidobacterium Triple Viable Enteric-Coated Capsules Combined with Enteral Nutrition on Patients with Chronic Critical Illness and Influence on Immune and Coagulation Function.Evid Based Complement Alternat Med. 2023 Dec 13;2023:9835982. doi: 10.1155/2023/9835982. eCollection 2023. Evid Based Complement Alternat Med. 2023. PMID: 38125195 Free PMC article.
-
Hypercatabolism and Anti-catabolic Therapies in the Persistent Inflammation, Immunosuppression, and Catabolism Syndrome.Front Nutr. 2022 Jul 13;9:941097. doi: 10.3389/fnut.2022.941097. eCollection 2022. Front Nutr. 2022. PMID: 35911117 Free PMC article. Review.
-
Dietary High Glycinin Reduces Growth Performance and Impairs Liver and Intestinal Health Status of Orange-Spotted Grouper (Epinephelus coioides).Animals (Basel). 2023 Aug 12;13(16):2605. doi: 10.3390/ani13162605. Animals (Basel). 2023. PMID: 37627396 Free PMC article.
-
Influence of early enteral nutrition plus probiotics on intestinal function of senile patients with sepsis.Am J Transl Res. 2023 Jan 15;15(1):445-451. eCollection 2023. Am J Transl Res. 2023. PMID: 36777858 Free PMC article.
References
Publication types
LinkOut - more resources
Full Text Sources
Research Materials

