Rosiglitazone Suppresses Renal Crystal Deposition by Ameliorating Tubular Injury Resulted from Oxidative Stress and Inflammatory Response via Promoting the Nrf2/HO-1 Pathway and Shifting Macrophage Polarization
- PMID: 34691355
- PMCID: PMC8531781
- DOI: 10.1155/2021/5527137
Rosiglitazone Suppresses Renal Crystal Deposition by Ameliorating Tubular Injury Resulted from Oxidative Stress and Inflammatory Response via Promoting the Nrf2/HO-1 Pathway and Shifting Macrophage Polarization
Abstract
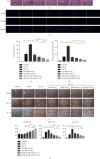
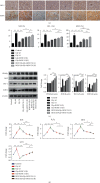
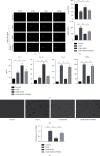
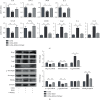
Oxidative stress and inflammatory response are closely related to nephrolithiasis. This study is aimed at exploring whether rosiglitazone (ROSI), a regulator of macrophage (Mp) polarization, could reduce renal calcium oxalate (CaOx) deposition by ameliorating oxidative stress and inflammatory response. Male C57 mice were equally and randomly divided into 7 groups. Kidney sections were collected on day 5 or day 8 after treatment. Pizzolato staining and polarized light optical microscopy were used to detect crystal deposition. PAS staining and TUNEL assay were performed to assess the tubular injury and cell apoptosis, respectively. Gene expression was assessed by immunohistochemistry, immunofluorescence, ELISA, qRT-PCR, and Western blot. The reactive oxygen species (ROS) level was assessed using a fluorescence microplate and fluorescence microscope. Hydrogen peroxide (H2O2), malonaldehyde (MDA), and glutathione (GSH) were evaluated to determine oxidative stress. Lactic dehydrogenase (LDH) activity was examined to detect cell injury. Adhesion of CaOx monohydrate (COM) crystals to HK-2 cells was detected by crystal adhesion assay. HK-2 cell death or renal macrophage polarization was assessed by flow cytometry. In vivo, renal crystal deposition, tubular injury, crystal adhesion, cell apoptosis, oxidative stress, and inflammatory response were significantly increased in the 7-day glyoxylic acid- (Gly-) treated group but were decreased in the ROSI-treated groups, especially in the groups pretreated with ROSI. Moreover, ROSI significantly reduced renal Mp aggregation and M1Mp polarization but significantly enhanced renal M2Mp polarization. In vitro, ROSI significantly suppressed renal injury, apoptosis, and crystal adhesion of HK-2 cells and markedly shifted COM-stimulated M1Mps to M2Mps, presenting an anti-inflammatory effect. Furthermore, ROSI significantly suppressed oxidative stress by promoting the Nrf2/HO-1 pathway in HK-2 cells. These findings indicate that ROSI could ameliorate renal tubular injury that resulted from oxidative stress and inflammatory response by suppressing M1Mp polarization and promoting M2Mp polarization. Therefore, ROSI is a potential therapeutic and preventive drug for CaOx nephrolithiasis.
Copyright © 2021 Hongyan Lu et al.
Conflict of interest statement
The authors declare no conflicts of interest regarding the publication of this paper.
Figures




References
-
- Neisius A., Preminger G. M. Stones in 2012: epidemiology, prevention and redefining therapeutic standards . - PubMed
-
- Antonelli J. A., Maalouf N. M., Pearle M. S., Lotan Y. Use of the National Health and Nutrition Examination Survey to calculate the impact of obesity and diabetes on cost and prevalence of urolithiasis in 2030. European Urology . 2014;66(4):724–729. doi: 10.1016/j.eururo.2014.06.036. - DOI - PMC - PubMed
-
- Khan S. R., Byer K. J., Thamilselvan S., et al. Crystal-cell interaction and apoptosis in oxalate-associated injury of renal epithelial cells. Journal of the American Society of Nephrology . 1999;10(Supplement 14):S457–S463. - PubMed
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Medical

