Direct transformation of dinitrogen: synthesis of N-containing organic compounds via N-C bond formation
- PMID: 34691489
- PMCID: PMC8288816
- DOI: 10.1093/nsr/nwaa142
Direct transformation of dinitrogen: synthesis of N-containing organic compounds via N-C bond formation
Abstract
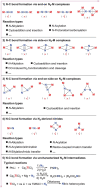
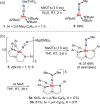
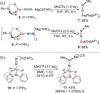
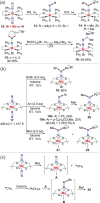
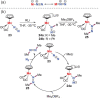
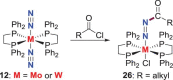
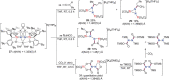
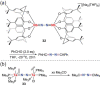
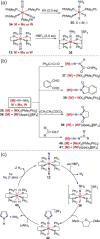
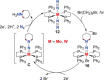
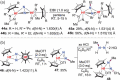
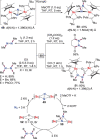
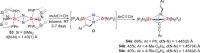
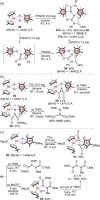
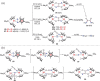
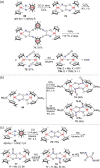
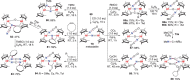
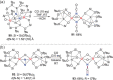
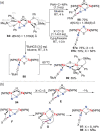
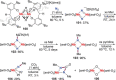
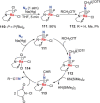
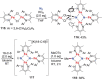
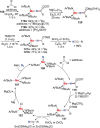
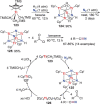
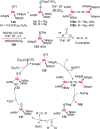
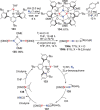
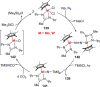
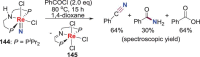
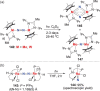
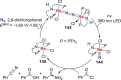
N-containing organic compounds are of vital importance to lives. Practical synthesis of valuable N-containing organic compounds directly from dinitrogen (N2), not through ammonia (NH3), is a holy-grail in chemistry and chemical industry. An essential step for this transformation is the functionalization of the activated N2 units/ligands to generate N-C bonds. Pioneering works of transition metal-mediated direct conversion of N2 into organic compounds via N-C bond formation at metal-dinitrogen [N2-M] complexes have generated diversified coordination modes and laid the foundation of understanding for the N-C bond formation mechanism. This review summarizes those major achievements and is organized by the coordination modes of the [N2-M] complexes (end-on, side-on, end-on-side-on, etc.) that are involved in the N-C bond formation steps, and each part is arranged in terms of reaction types (N-alkylation, N-acylation, cycloaddition, insertion, etc.) between [N2-M] complexes and carbon-based substrates. Additionally, earlier works on one-pot synthesis of organic compounds from N2 via ill-defined intermediates are also briefed. Although almost all of the syntheses of N-containing organic compounds via direct transformation of N2 so far in the literature are realized in homogeneous stoichiometric thermochemical reaction systems and are discussed here in detail, the sporadically reported syntheses involving photochemical, electrochemical, heterogeneous thermo-catalytic reactions, if any, are also mentioned. This review aims to provide readers with an in-depth understanding of the state-of-the-art and perspectives of future research particularly in direct catalytic and efficient conversion of N2 into N-containing organic compounds under mild conditions, and to stimulate more research efforts to tackle this long-standing and grand scientific challenge.
Keywords: N-containing organic compounds; N−C bond formation; dinitrogen transformation; metal-dinitrogen complex.
© The Author(s) 2020. Published by Oxford University Press on behalf of China Science Publishing & Media Ltd.
Figures
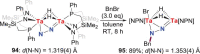
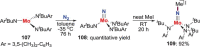

References
-
- Walter MD. Recent advances in transition metal-catalyzed dinitrogen activation. In: Pedro JP (ed.). Advances in Organometallic Chemistry. New York:Academic Press, 2016, vol. 65, Chapter 5: 261–377.
LinkOut - more resources
Full Text Sources
