Diffusion and catalyst efficiency in hierarchical zeolite catalysts
- PMID: 34691504
- PMCID: PMC8290962
- DOI: 10.1093/nsr/nwaa184
Diffusion and catalyst efficiency in hierarchical zeolite catalysts
Abstract
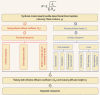
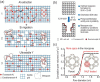
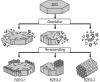
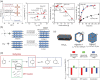
The preparation of hierarchical zeolites with reduced diffusion limitation and enhanced catalyst efficiency has become a vital focus in the field of zeolites and porous materials chemistry within the past decades. This review will focus on the diffusion and catalyst efficiency of hierarchical zeolites and industrial catalysts. The benefits of diffusion and catalyst efficiency at two levels of hierarchies (zeolitic component level and industrial catalyst level) from a chemical reaction engineering point of view will be analysed. At zeolitic component level, three types of mesopores based on the strategies applied toward enhancing the catalyst effectiveness factor are presented: (i) 'functional mesopores' (raising effective diffusivity); (ii) 'auxiliary mesopores' (decreasing diffusion length); and (iii) 'integrated mesopores' (a combination thereof). At industrial catalyst level, location and interconnectivity among the constitutive components are revealed. The hierarchical pore interconnectivity in multi-component zeolite based industrial catalysts is exemplified by fluid catalytic cracking and bi-functional hydroisomerization catalysts. The rational design of industrial zeolite catalysts at both hierarchical zeolitic component and catalyst body levels can be fully comprehended using the advanced in situ and/or operando spectroscopic, microscopic and diffraction techniques.
Keywords: advanced characterization; diffusion; effectiveness factor; hierarchical zeolite; industrial catalyst; pore connectivity.
© The Author(s) 2020. Published by Oxford University Press on behalf of China Science Publishing & Media Ltd.
Figures







Similar articles
-
Revealing the Crucial Roles of Pore Interconnectivity between Zeolitic and Nonzeolitic Components in Enhancing Diffusion and Catalytic Efficiency of Industrial Zeolite-Based Catalysts.J Am Chem Soc. 2025 Jun 4;147(22):18550-18562. doi: 10.1021/jacs.5c00214. Epub 2025 May 26. J Am Chem Soc. 2025. PMID: 40419460
-
Molecular transport in zeolite catalysts: depicting an integrated picture from macroscopic to microscopic scales.Chem Soc Rev. 2022 Oct 3;51(19):8174-8200. doi: 10.1039/d2cs00079b. Chem Soc Rev. 2022. PMID: 36069165 Review.
-
Recent advances of pore system construction in zeolite-catalyzed chemical industry processes.Chem Soc Rev. 2015 Dec 21;44(24):8877-903. doi: 10.1039/c5cs00626k. Epub 2015 Nov 16. Chem Soc Rev. 2015. PMID: 26567526
-
Zeolitic materials with hierarchical porous structures.Adv Mater. 2011 Jun 17;23(22-23):2602-15. doi: 10.1002/adma.201100462. Epub 2011 Apr 15. Adv Mater. 2011. PMID: 21495091
-
Advances in the Synthesis of Ferrierite Zeolite.Molecules. 2020 Aug 14;25(16):3722. doi: 10.3390/molecules25163722. Molecules. 2020. PMID: 32824105 Free PMC article. Review.
Cited by
-
Boosting molecular diffusion following the generalized Murray's Law by constructing hierarchical zeolites for maximized catalytic activity.Natl Sci Rev. 2022 Oct 27;9(12):nwac236. doi: 10.1093/nsr/nwac236. eCollection 2022 Dec. Natl Sci Rev. 2022. PMID: 36632521 Free PMC article.
-
Hierarchy in materials for maximized efficiency.Natl Sci Rev. 2020 Sep 26;7(11):1626-1630. doi: 10.1093/nsr/nwaa251. eCollection 2020 Nov. Natl Sci Rev. 2020. PMID: 34691495 Free PMC article.
-
Advanced TiO2-Based Photocatalytic Systems for Water Splitting: Comprehensive Review from Fundamentals to Manufacturing.Molecules. 2025 Feb 28;30(5):1127. doi: 10.3390/molecules30051127. Molecules. 2025. PMID: 40076350 Free PMC article. Review.
-
Enhanced Adsorption of Trace Ethylene on Ag/NZ5 Modified with Ammonia: Hierarchical Structure and Metal Dispersion Effects.Molecules. 2024 Feb 23;29(5):981. doi: 10.3390/molecules29050981. Molecules. 2024. PMID: 38474493 Free PMC article.
-
Targeted synthesis of zeolites from calculated interaction between zeolite structure and organic template.Natl Sci Rev. 2022 Feb 23;9(9):nwac023. doi: 10.1093/nsr/nwac023. eCollection 2022 Sep. Natl Sci Rev. 2022. PMID: 36128457 Free PMC article. Review.
References
-
- Cejka J, Van Bekkum H, Corma A et al. . Introduction to Zeolite Molecular Sieves. Amsterdam: Elsevier Science, 2007.
-
- Martínez C, Corma A. Inorganic molecular sieves: preparation, modification and industrial application in catalytic processes. Coord Chem Rev 2011; 255: 1558–80.10.1016/j.ccr.2011.03.014 - DOI
-
- Gilson J-P, Vermeiren W. Impact of zeolites on the petroleum and petrochemical industry. Top Catal 2009; 52: 1131–61.10.1007/s11244-009-9271-8 - DOI
-
- Al-Khattaf S, de Lasa H. Activity and selectivity of fluidized catalytic cracking catalysts in a riser simulator: the role of Y-zeolite crystal size. Ind Eng Chem Res 1999; 38: 1350–6.10.1021/ie980433z - DOI
-
- Al-Sabawi M, Atias JA, de Lasa H. Heterogeneous approach to the catalytic cracking of vacuum gas oil. Ind Eng Chem Res 2008; 47: 7631–41.10.1021/ie701745k - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
