Immobilization of functional nano-objects in living engineered bacterial biofilms for catalytic applications
- PMID: 34691954
- PMCID: PMC8291418
- DOI: 10.1093/nsr/nwz104
Immobilization of functional nano-objects in living engineered bacterial biofilms for catalytic applications
Abstract
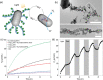
Nanoscale objects feature very large surface-area-to-volume ratios and are now understood as powerful tools for catalysis, but their nature as nanomaterials brings challenges including toxicity and nanomaterial pollution. Immobilization is considered a feasible strategy for addressing these limitations. Here, as a proof-of-concept for the immobilization of nanoscale catalysts in the extracellular matrix of bacterial biofilms, we genetically engineered amyloid monomers of the Escherichia coli curli nanofiber system that are secreted and can self-assemble and anchor nano-objects in a spatially precise manner. We demonstrated three scalable, tunable and reusable catalysis systems: biofilm-anchored gold nanoparticles to reduce nitro aromatic compounds such as the pollutant p-nitrophenol, biofilm-anchored hybrid Cd0.9Zn0.1S quantum dots and gold nanoparticles to degrade organic dyes and biofilm-anchored CdSeS@ZnS quantum dots in a semi-artificial photosynthesis system for hydrogen production. Our work demonstrates how the ability of biofilms to grow in scalable and complex spatial arrangements can be exploited for catalytic applications and clearly illustrates the design utility of segregating high-energy nano-objects from injury-prone cellular components by engineering anchoring points in an extracellular matrix.
Keywords: bio-inorganic hybrid system; hydrogen production; living catalysis; nanoscale catalyst immobilization; semi-artificial photosynthesis.
© The Author(s) 2019. Published by Oxford University Press on behalf of China Science Publishing & Media Ltd.
Figures



Similar articles
-
Engineered catalytic biofilms: Site-specific enzyme immobilization onto E. coli curli nanofibers.Biotechnol Bioeng. 2015 Oct;112(10):2016-24. doi: 10.1002/bit.25638. Epub 2015 May 20. Biotechnol Bioeng. 2015. PMID: 25950512
-
Programming Cells for Dynamic Assembly of Inorganic Nano-Objects with Spatiotemporal Control.Adv Mater. 2018 Apr;30(16):e1705968. doi: 10.1002/adma.201705968. Epub 2018 Mar 8. Adv Mater. 2018. PMID: 29516606
-
Synthetic Biogenesis of Bacterial Amyloid Nanomaterials with Tunable Inorganic-Organic Interfaces and Electrical Conductivity.ACS Synth Biol. 2017 Feb 17;6(2):266-275. doi: 10.1021/acssynbio.6b00166. Epub 2016 Dec 6. ACS Synth Biol. 2017. PMID: 27794590 Free PMC article.
-
Engineered tyrosinases with broadened bio-catalysis scope: immobilization using nanocarriers and applications.3 Biotech. 2021 Aug;11(8):365. doi: 10.1007/s13205-021-02913-6. Epub 2021 Jul 5. 3 Biotech. 2021. PMID: 34290948 Free PMC article. Review.
-
Synthetic biology engineering of biofilms as nanomaterials factories.Biochem Soc Trans. 2017 Jun 15;45(3):585-597. doi: 10.1042/BST20160348. Biochem Soc Trans. 2017. PMID: 28620023 Review.
Cited by
-
Encapsulation of bacterial cells in cytoprotective ZIF-90 crystals as living composites.Mater Today Bio. 2021 Feb 4;10:100097. doi: 10.1016/j.mtbio.2021.100097. eCollection 2021 Mar. Mater Today Bio. 2021. PMID: 33733083 Free PMC article.
-
Preparation and biomedical applications of artificial cells.Mater Today Bio. 2023 Nov 24;23:100877. doi: 10.1016/j.mtbio.2023.100877. eCollection 2023 Dec. Mater Today Bio. 2023. PMID: 38075249 Free PMC article. Review.
-
Photocatalytic Material-Microorganism Hybrid System and Its Application-A Review.Micromachines (Basel). 2022 May 30;13(6):861. doi: 10.3390/mi13060861. Micromachines (Basel). 2022. PMID: 35744475 Free PMC article. Review.
-
Encoding with a fluorescence-activating and absorption-shifting tag generates living bacterial probes for mammalian microbiota imaging.Mater Today Bio. 2022 Jun 6;15:100311. doi: 10.1016/j.mtbio.2022.100311. eCollection 2022 Jun. Mater Today Bio. 2022. PMID: 35711290 Free PMC article.
-
Rational Design of Artificial Biofilms as Sustainable Supports for Whole-Cell Catalysis Through Integrating Extra- and Intracellular Catalysis.ChemSusChem. 2022 Sep 7;15(17):e202200850. doi: 10.1002/cssc.202200850. Epub 2022 Jul 13. ChemSusChem. 2022. PMID: 35726119 Free PMC article.
References
-
- times Challenging. Nat Nanotechnol 2016; 11: 397. - PubMed
-
- Li Q and Sun SH. Recent advances in the organic solution phase synthesis of metal nanoparticles and their electrocatalysis for energy conversion reactions. Nano Energy 2016; 29:178–97.
-
- Han Z, Qiu F and Eisenberg Ret al. . Robust photogeneration of H2 in water using semiconductor nanocrystals and a nickel catalyst. Science 2012; 338: 1321–4. - PubMed
-
- Chemistry Hashmi AS.. Sub-nanosized gold catalysts. Science 2012; 338: 1434. - PubMed
-
- Mistry H, Varela AS and Kuhl Set al. . Nanostructured electrocatalysts with tunable activity and selectivity. Nat Rev Mater 2016; 1: 16009.
LinkOut - more resources
Full Text Sources
