High-resolution mapping of brain vasculature and its impairment in the hippocampus of Alzheimer's disease mice
- PMID: 34692000
- PMCID: PMC8291402
- DOI: 10.1093/nsr/nwz124
High-resolution mapping of brain vasculature and its impairment in the hippocampus of Alzheimer's disease mice
Abstract
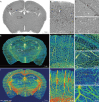
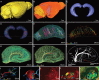
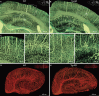
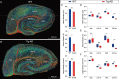
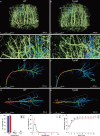
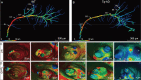
Accumulating evidence indicates the critical importance of cerebrovascular dysfunction in the pathogenesis of Alzheimer's disease (AD). However, systematic comparative studies on the precise brain vasculature of wild-type and AD model mice are still rare. Using an image-optimization method for analysing Micro-Optical Sectioning Tomography (MOST) data, we generated cross-scale whole-brain 3D atlases that cover the entire vascular system from large vessels down to smallest capillaries at submicron resolution, for both wild-type mice and a transgenic (APP/PS1) mouse model of AD. In addition to distinct vascular patterns in different brain regions, we found that the main vessels of the molecular layer of the hippocampal dentate gyrus (DG-ml) undergo abrupt changes in both diameter and branch angle, spreading a unique comb-like pattern of capillaries. By using a quantitative analysis workflow, we identified in the hippocampus of AD mice an overall reduction of the mean vascular diameter, volume fraction and branch angle, with most significant impairment in the DG-ml. In addition, virtual endoscopy revealed irregular morphological features in the vessel lumen of the AD mice, potentially contributing to the impairment of blood flow. Our results demonstrate the capability of high-resolution cross-scale evaluation of brain vasculature and underscore the importance of studying hippocampal microcirculation for understanding AD pathogenesis.
Keywords: Alzheimer's disease; Micro-Optical Sectioning Tomography; image optimization; impaired hippocampal vasculature; virtual vascular endoscopy.
© The Author(s) 2019. Published by Oxford University Press on behalf of China Science Publishing & Media Ltd.
Figures
References
-
- Martin P, Anders W, Maëlenn G. World Alzheimer report. In: the global impact of dementia. Alzheimer's Disease International. London: ADI, 2015.
-
- Sugino H, Watanabe A, Amada Net al. Global trends in Alzheimer disease clinical development: increasing the probability of success. Clin Ther 2015; 37: 1632–42. - PubMed
-
- Wang YJ. Lessons from immunotherapy for Alzheimer disease. Nat Rev Neurol 2014; 10: 188–9. - PubMed
-
- Sharma P, Srivastava P, Seth Aet al. Comprehensive review of mechanisms of pathogenesis involved in Alzheimer's disease and potential therapeutic strategies. Prog Neurobiol 2019; 174: 53–89. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
