Potential-tuned selective electrosynthesis of azoxy-, azo- and amino-aromatics over a CoP nanosheet cathode
- PMID: 34692044
- PMCID: PMC8288891
- DOI: 10.1093/nsr/nwz146
Potential-tuned selective electrosynthesis of azoxy-, azo- and amino-aromatics over a CoP nanosheet cathode
Abstract
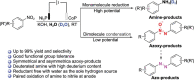
Azoxy-, azo- and amino-aromatics are among the most widely used building blocks in materials science pharmaceuticals and synthetic chemistry, but their controllable and green synthesis has not yet been well established. Herein, a facile potential-tuned electrosynthesis of azoxy-, azo- and amino-aromatics via aqueous selective reduction of nitroarene feedstocks over a CoP nanosheet cathode is developed. A series of azoxy-, azo- and amino-compounds with excellent selectivity, good functional group tolerance and high yields are produced by applying different bias input. The synthetically significant and challenging asymmetric azoxy-aromatics can be controllably synthesized in moderate to good yields. The use of water as the hydrogen source makes this strategy remarkably fascinating and promising. In addition, deuterated aromatic amines with a high deuterium content can be readily obtained by using D2O. By pairing with anodic oxidation of aliphatic amines to nitriles, synthetically useful building blocks can be simultaneously produced in a CoP||Ni2P two-electrode electrolyzer. Only 1.25 V is required to achieve a current density of 20 mA cm-2, which is much lower than that of overall water splitting (1.70 V). The paired oxidation and reduction reactions can also be driven using a 1.5 V battery to synthesize nitrile and azoxybenzene with good yields and selectivity, further emphasizing the flexibility and controllability of our method. This work paves the way for a promising approach to the green synthesis of valuable chemicals through potential-controlled electrosynthesis.
Keywords: azo- and amino-aromatics; azoxy; electrosynthesis; metal phosphides; nitroarenes; selectivity.
© The Author(s) 2019. Published by Oxford University Press on behalf of China Science Publishing & Media Ltd.
Figures



References
-
- Formenti D, Ferretti F, Scharnagl FKet al. . Reduction of nitro compounds using 3d-non-noble metal catalysts. Chem Rev 2019; 119: 2611–80. - PubMed
-
- Belowicha ME, Stoddart JF. Dynamic imine chemistry. Chem Soc Rev 2012; 41: 2003–24. - PubMed
-
- Jagadeesh RV, Murugesan K, Alshammari ASet al. . MOF-derived cobalt nanoparticles catalyze a general synthesis of amines. Science 2017; 358: 326–32. - PubMed
-
- Li J, Song S, Long Yet al. . Investigating the hybrid-structure-effect of CeO2-encapsulated Au nanostructures on the transfer coupling of nitrobenzene. Adv Mater 2018; 30: 1704416. - PubMed
-
- Grirrane A, Corma A, García H. Gold-catalyzed synthesis of aromatic azo compounds from anilines and nitroaromatics. Science 2008; 322: 1661–4. - PubMed
LinkOut - more resources
Full Text Sources
