Enantioselective assembly of multi-layer 3D chirality
- PMID: 34692078
- PMCID: PMC8289020
- DOI: 10.1093/nsr/nwz203
Enantioselective assembly of multi-layer 3D chirality
Abstract
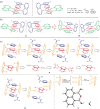
The first enantioselective assembly of sandwich-shaped organo molecules has been achieved by conducting dual asymmetric Suzuki-Miyaura couplings and nine other reactions. This work also presents the first fully C-C anchored multi-layer 3D chirality with optically pure enantiomers. As confirmed by X-ray diffraction analysis that this chiral framework is featured by a unique C2 -symmetry in which a nearly parallel fashion consisting of three layers: top, middle and bottom aromatic rings. Unlike the documented planar or axial chirality, the present chirality shows its top and bottom layers restrict each other from free rotation, i.e., this multi-layer 3D chirality would not exist if either top or bottom layer is removed. Nearly all multi-layered compounds showed strong luminescence of different colors under UV irradiation, and several randomly selected samples displayed aggregation-induced emission (AIE) properties. This work is believed to have broad impacts on chemical, medicinal and material sciences including optoelectronic materials in future.
Keywords: C2-symmetry; aggregation-induced emission (AIE); architecture chirality; multi-layer 3D chirality; multi-layered organic framework (M-LOF); organo sandwich chirality.
© The Author(s) 2019. Published by Oxford University Press on behalf of China Science Publishing & Media Ltd.
Figures





Similar articles
-
Central-to-Folding Chirality Control: Asymmetric Synthesis of Multilayer 3D Targets With Electron-Deficient Bridges.Front Chem. 2022 Mar 31;10:860398. doi: 10.3389/fchem.2022.860398. eCollection 2022. Front Chem. 2022. PMID: 35433628 Free PMC article.
-
Asymmetric Catalytic Approach to Multilayer 3D Chirality.Chemistry. 2021 May 26;27(30):8013-8020. doi: 10.1002/chem.202100700. Epub 2021 May 6. Chemistry. 2021. PMID: 33830589
-
Multilayer 3D Chirality and Its Synthetic Assembly.Research (Wash D C). 2019 Jun 27;2019:6717104. doi: 10.34133/2019/6717104. eCollection 2019. Research (Wash D C). 2019. PMID: 31549078 Free PMC article.
-
Chiral assembly of organic luminogens with aggregation-induced emission.Chem Sci. 2021 Jun 10;13(3):611-632. doi: 10.1039/d1sc02305e. eCollection 2022 Jan 19. Chem Sci. 2021. PMID: 35173927 Free PMC article. Review.
-
Pillararenes: fascinating planar chiral macrocyclic arenes.Chem Commun (Camb). 2021 Sep 9;57(72):9029-9039. doi: 10.1039/d1cc03778a. Chem Commun (Camb). 2021. PMID: 34498646 Review.
Cited by
-
Recent Advances in π-Stacking Interaction-Controlled Asymmetric Synthesis.Molecules. 2024 Mar 24;29(7):1454. doi: 10.3390/molecules29071454. Molecules. 2024. PMID: 38611737 Free PMC article. Review.
-
Orientational Chirality, Its Asymmetric Control, and Computational Study.Research (Wash D C). 2022 Dec 19;2022:0012. doi: 10.34133/research.0012. eCollection 2022. Research (Wash D C). 2022. PMID: 39290963 Free PMC article.
-
Multilayer 3D Chiral Folding Polymers and Their Asymmetric Catalytic Assembly.Research (Wash D C). 2022 Feb 16;2022:9847949. doi: 10.34133/2022/9847949. eCollection 2022. Research (Wash D C). 2022. PMID: 35265849 Free PMC article.
-
Organocatalytic Asymmetric [2 + 4] Cycloadditions of 3-Vinylindoles with ortho-Quinone Methides.Molecules. 2021 Nov 8;26(21):6751. doi: 10.3390/molecules26216751. Molecules. 2021. PMID: 34771158 Free PMC article.
-
Central-to-Folding Chirality Control: Asymmetric Synthesis of Multilayer 3D Targets With Electron-Deficient Bridges.Front Chem. 2022 Mar 31;10:860398. doi: 10.3389/fchem.2022.860398. eCollection 2022. Front Chem. 2022. PMID: 35433628 Free PMC article.
References
-
- Eliel EL, Wilen SH. Stereochemistry of Organic Compounds. New York: John Wiley & Sons, 1994, 1172–5.
-
- Wagner I, Musso H. New naturally occurring amino acids. Angew Chem Int Ed 1983; 22: 816–28.
-
- William RM. Synthesis of Optically Active α‐Amino Acids. Oxford: Pergamon Press, 1990.
-
- Dunitz JD. Pauling's left‐handed α‐helix. Angew Chem Int Ed 2001; 40: 4167–73. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
