Glucocorticoid-Responsive Transcription Factor Krüppel-Like Factor 9 Regulates fkbp5 and Metabolism
- PMID: 34692682
- PMCID: PMC8526736
- DOI: 10.3389/fcell.2021.727037
Glucocorticoid-Responsive Transcription Factor Krüppel-Like Factor 9 Regulates fkbp5 and Metabolism
Abstract
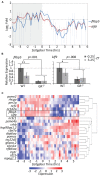
Krüppel-like factor 9 (Klf9) is a feedforward regulator of glucocorticoid receptor (GR) signaling. Here we show that in zebrafish klf9 is expressed with GR-dependent oscillatory dynamics in synchrony with fkbp5, a GR target that encodes a negative feedback regulator of GR signaling. We found that fkbp5 transcript levels are elevated in klf9 -/- mutants and that Klf9 associates with chromatin at the fkbp5 promoter, which becomes hyperacetylated in klf9 -/ - mutants, suggesting that the GR regulates fkbp5 via an incoherent feedforward loop with klf9. As both the GR and Fkbp5 are known to regulate metabolism, we asked how loss of Klf9 affects metabolic rate and gene expression. We found that klf9 -/- mutants have a decreased oxygen consumption rate (OCR) and upregulate glycolytic genes, the promoter regions of which are enriched for potential Klf9 binding motifs. Our results suggest that Klf9 functions downstream of the GR to regulate cellular glucocorticoid responsivity and metabolic homeostasis.
Keywords: Krüppel-like factor 9; RNA-seq; cortisol; fkbp5; gene expression; glucocorticoid receptor; metabolism; zebrafish.
Copyright © 2021 Gans, Grendler, Babich, Jayasundara and Coffman.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



References
-
- Alon U. (2019). An Introduction to Systems Biology: Design Principles of Biological Circuits, 2nd Edn. London: Chapman and Hall. 10.1201/9780429283321 - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

