The Role of MicroRNAs in Therapeutic Resistance of Malignant Primary Brain Tumors
- PMID: 34692698
- PMCID: PMC8529124
- DOI: 10.3389/fcell.2021.740303
The Role of MicroRNAs in Therapeutic Resistance of Malignant Primary Brain Tumors
Abstract
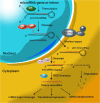
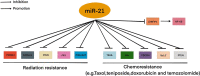
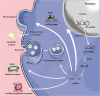
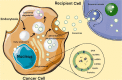
Brain tumors in children and adults are challenging tumors to treat. Malignant primary brain tumors (MPBTs) such as glioblastoma have very poor outcomes, emphasizing the need to better understand their pathogenesis. Developing novel strategies to slow down or even stop the growth of brain tumors remains one of the major clinical challenges. Modern treatment strategies for MPBTs are based on open surgery, chemotherapy, and radiation therapy. However, none of these treatments, alone or in combination, are considered effective in controlling tumor progression. MicroRNAs (miRNAs) are 18-22 nucleotide long endogenous non-coding RNAs that regulate gene expression at the post-transcriptional level by interacting with 3'-untranslated regions (3'-UTR) of mRNA-targets. It has been proven that miRNAs play a significant role in various biological processes, including the cell cycle, apoptosis, proliferation, differentiation, etc. Over the last decade, there has been an emergence of a large number of studies devoted to the role of miRNAs in the oncogenesis of brain tumors and the development of resistance to radio- and chemotherapy. Wherein, among the variety of molecules secreted by tumor cells into the external environment, extracellular vesicles (EVs) (exosomes and microvesicles) play a special role. Various elements were found in the EVs, including miRNAs, which can be transported as part of these EVs both between neighboring cells and between remotely located cells of different tissues using biological fluids. Some of these miRNAs in EVs can contribute to the development of resistance to radio- and chemotherapy in MPBTs, including multidrug resistance (MDR). This comprehensive review examines the role of miRNAs in the resistance of MPBTs (e.g., high-grade meningiomas, medulloblastoma (MB), pituitary adenomas (PAs) with aggressive behavior, and glioblastoma) to chemoradiotherapy and pharmacological treatment. It is believed that miRNAs are future therapeutic targets in MPBTs and such the role of miRNAs needs to be critically evaluated to focus on solving the problems of resistance to therapy this kind of human tumors.
Keywords: exosomes; malignant primary brain tumors; miRNAs; oncogenesis; resistance; therapy.
Copyright © 2021 Gareev, Beylerli, Liang, Xiang, Liu, Xu, Yuan, Ahmad and Yang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

