Extracellular Vesicles in Acute Leukemia: A Mesmerizing Journey With a Focus on Transferred microRNAs
- PMID: 34692712
- PMCID: PMC8527035
- DOI: 10.3389/fcell.2021.766371
Extracellular Vesicles in Acute Leukemia: A Mesmerizing Journey With a Focus on Transferred microRNAs
Abstract
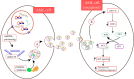
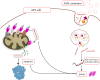
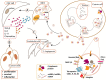
Despite their small size, the membrane-bound particles named extracellular vesicles (EVs) seem to play an enormous role in the pathogenesis of acute leukemia. From oncogenic hematopoietic stem cells (HSCs) to become leukemic cells to alter the architecture of bone marrow (BM) microenvironment, EVs are critical components of leukemia development. As a carrier of essential molecules, especially a group of small non-coding RNAs known as miRNA, recently, EVs have attracted tremendous attention as a prognostic factor. Given the importance of miRNAs in the early stages of leukemogenesis and also their critical parts in the development of drug-resistant phenotype, it seems that the importance of EVs in the development of leukemia is more than what is expected. To be familiar with the clinical value of leukemia-derived EVs, this review aimed to briefly shed light on the biology of EVs and to discuss the role of EV-derived miRNAs in the development of acute myeloid leukemia and acute lymphoblastic leukemia. By elaborating the advances and challenges concerning the isolation of EVs, we discuss whether EVs could have a prognostic value in the clinical setting for leukemia.
Keywords: acute lymphoblastic leukemia; acute myeloblastic leukemia; disease pathogenesis; extracellular vesicles; miRNAs; non-coding RNAs; prognosis factor.
Copyright © 2021 Izadirad, Huang, Jafari, Hamidieh, Gharehbaghian, Li, Jafari and Chen.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Role of Acute Myeloid Leukemia (AML)-Derived exosomes in tumor progression and survival.Biomed Pharmacother. 2022 Jun;150:113009. doi: 10.1016/j.biopha.2022.113009. Epub 2022 Apr 26. Biomed Pharmacother. 2022. PMID: 35486974 Review.
-
AML-derived extracellular vesicles negatively regulate stem cell pool size: A step toward bone marrow failure.Curr Res Transl Med. 2023 Jan-Mar;71(1):103375. doi: 10.1016/j.retram.2022.103375. Epub 2022 Nov 30. Curr Res Transl Med. 2023. PMID: 36508911
-
Extracellular vesicles in acute myeloid leukemia: The role in disease pathogenesis, potential biomarker, and application in clinical settings.Crit Rev Oncol Hematol. 2025 Jul;211:104743. doi: 10.1016/j.critrevonc.2025.104743. Epub 2025 Apr 23. Crit Rev Oncol Hematol. 2025. PMID: 40280220 Review.
-
Comparative analysis of extracellular vesicle isolation methods from human AML bone marrow cells and AML cell lines.Front Oncol. 2022 Oct 3;12:949261. doi: 10.3389/fonc.2022.949261. eCollection 2022. Front Oncol. 2022. PMID: 36263223 Free PMC article.
-
The miRNA Content of Bone Marrow-Derived Extracellular Vesicles Contributes to Protein Pathway Alterations Involved in Ionising Radiation-Induced Bystander Responses.Int J Mol Sci. 2023 May 11;24(10):8607. doi: 10.3390/ijms24108607. Int J Mol Sci. 2023. PMID: 37239971 Free PMC article.
Cited by
-
'One way, or another, I'm gonna find ya': miR-221-3p finds its targets via small extracellular vesicles.Haematologica. 2024 Oct 1;109(10):3094-3096. doi: 10.3324/haematol.2024.285257. Haematologica. 2024. PMID: 38634128 Free PMC article. No abstract available.
-
Dual roles of extracellular vesicles in acute lymphoblastic leukemia: implications for disease progression and theranostic strategies.Med Oncol. 2024 Nov 22;42(1):11. doi: 10.1007/s12032-024-02547-7. Med Oncol. 2024. PMID: 39572459 Free PMC article. Review.
-
Cell Type-Specific Extracellular Vesicles and Their Impact on Health and Disease.Int J Mol Sci. 2024 Feb 27;25(5):2730. doi: 10.3390/ijms25052730. Int J Mol Sci. 2024. PMID: 38473976 Free PMC article. Review.
-
ActivinA modulates B-acute lymphoblastic leukaemia cell communication and survival by inducing extracellular vesicles production.Sci Rep. 2024 Jul 12;14(1):16083. doi: 10.1038/s41598-024-66779-3. Sci Rep. 2024. PMID: 38992199 Free PMC article.
-
Circular RNAs: pivotal role in the leukemogenesis and novel indicators for the diagnosis and prognosis of acute myeloid leukemia.Front Oncol. 2023 Apr 14;13:1149187. doi: 10.3389/fonc.2023.1149187. eCollection 2023. Front Oncol. 2023. PMID: 37124518 Free PMC article. Review.
References
-
- Baranyai T., Herczeg K., Onódi Z., Voszka I., Módos K., Marton N., et al. (2015). Isolation of exosomes from blood plasma: qualitative and quantitative comparison of ultracentrifugation and size exclusion chromatography methods. PloS One 10:e0145686. 10.1371/journal.pone.0145686 - DOI - PMC - PubMed
-
- Barzegar M., Allahbakhshian Farsani M., Amiri V., Mohammadi S., Shahsavan S., Mirzaeian A., et al. (2021). AML-derived extracellular vesicles confer de novo chemoresistance to leukemic myeloblast cells by promoting drug export genes expression and ROS inhibition. Iran. J. Pharm. Res. 20 384–397. - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials

