Modulating OPG and TGF-β1 mRNA expression via bioelectrical stimulation
- PMID: 34692946
- PMCID: PMC8517839
- DOI: 10.1016/j.bonr.2021.101141
Modulating OPG and TGF-β1 mRNA expression via bioelectrical stimulation
Abstract
Background: Bone remodeling is a lifelong process that ranges from orthodontic tooth movement/alignment to bone damage/healing, to overall bone health. Osteoprotegerin (OPG) and transforming growth factor β1 (TGF-β1) are secreted by osteoblasts and participate in bone remodeling. OPG promotes bone remineralization and stabilization prominent in post-mechanical repositioning of the teeth in the dental alveolus. TGF-β1 participates in regulatory processes to promote osteoblast and osteoclast equilibrium. In the context of orthodontic tooth movement, post-treatment fixation requires additional, exogenous, stabilization support. Recent research showcases supplementary solutions, in conjunction to standard tooth fixation techniques, such as OPG injections into gum and periodontal tissues to accelerate tooth anchorage; however, injections are prone to post-procedure complications and discomfort. This study utilizes noninvasive bioelectric stimulation (BES) to modulate OPG and TGF-β1 as a novel solution to regulate bone remineralization specifically in the context of post-orthodontic tooth movement.
Purpose: The aim of this study was to investigate a spectrum of BES parameters that would modulate OPG and TGF-β1 expression in osteoblasts.
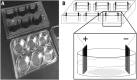
Methods: Osteoblasts were cultured and stimulated using frequencies from 25 Hz to 3 MHz. RT-qPCR was used to quantify changes in OPG and TGFb-1 mRNA expression.
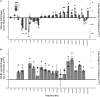
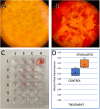
Results: OPG mRNA expression was significantly increased at frequencies above 10,000 Hz with a maximum expression increase of 332 ± 8% at 100 kHz. Conversely, OPG mRNA expression was downregulated at frequencies lower than 1000 Hz. TGF-β1 mRNA expression increased throughout all stimulation frequencies with a peak of 332 ± 72% at 250 kHz. Alizarin Red tests for calcium, indicated that mineralization of stimulated osteoblasts in vitro increased 28% after 6 weeks in culture.
Discussion: Results support the working hypothesis that OPG and TGF-β1 mRNA expression can be modulated through BES. Noninvasive BES approaches have the potential to accelerate bone remineralization by providing a novel tool to supplement the anchorage process, reduce complications, and promote patient compliance and reduce post-treatment relapse. Noninvasive BES may be applicable to other clinical applications as a novel therapeutic tool to modulate bone remodeling.
Keywords: Bone remodeling/regeneration; Electrophysiology; Gene expression; Growth factor; Mineralization in vitro; Orthodontic tooth movement; Osteoblast.
© 2021 Published by Elsevier Inc.
Figures


References
-
- Baud’huin M., Duplomb L., Teletchea S., Lamoureux F., Ruiz-Velasco C., Maillasson M., Redini F., Heymann M.-F., Heymann D. Osteoprotegerin: multiple partners for multiple functions. Cytokine Growth Factor Rev. 2013;24(5):401–409. - PubMed
-
- Baxter S.J., Sydorak I., Ma P.X., Hatch N.E. Impact of pharmacologic inhibition of tooth movement on periodontal and tooth root tissues during orthodontic force application. Orthod. Craniofacial Res. 2020;23(1):35–43. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

