Disclosure of individual research results at federally funded Alzheimer's Disease Research Centers
- PMID: 34692986
- PMCID: PMC8515553
- DOI: 10.1002/trc2.12213
Disclosure of individual research results at federally funded Alzheimer's Disease Research Centers
Abstract
Introduction: This study describes practices for disclosing individual research results to participants in Alzheimer's disease research.
Methods: An online survey of clinical core leaders at National Institutes of Health-funded Alzheimer's Disease Research Centers in the United States (response rate: 30/31, 97%) examined return of results practices across nine different types of research results.
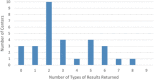
Results: Most centers had returned consensus research diagnoses (83%) and neuropsychological test results (73%), with fewer having shared amyloid positron emission tomography (43%), tau imaging (10%), or apolipoprotein E (APOE) genotype (7%) results. Centers reported having disclosed a mean of 3.1 types of results (standard deviation = 2.1; range 0-8). The most commonly cited reason for disclosure was to inform participants' medical decision-making (88%). Disclosure involved multiple professionals and modalities, with neurologists (87%) and in-person visits (85%) most commonplace.
Discussion: Centers varied widely as to whether and how they disclosed research results. Diagnostic and cognitive test results were more commonly returned than genetic or biomarker results.
Keywords: biomarkers; genetic testing; research ethics; return of research results; risk communication.
© 2021 The Authors. Alzheimer's & Dementia: Diagnosis, Assessment & Disease Monitoring published by Wiley Periodicals, LLC on behalf of Alzheimer's Association.
Conflict of interest statement
The authors report no financial conflicts of interest. Dr. Roberts has served as a paid advisory board member for the national Alzheimer's Clinical Trials Consortium and NIH‐funded research projects based at Indiana University (U01 AG057195), Washington University (R01AG065234), and Children's National Medical Center (R01 HD095068). He also serves in an unpaid role as co‐chair of the Michigan Dementia Coalition. Dr. Blacker and Ms. Rumbaugh serve in unpaid roles as co‐chairs of the Asymptomatic Subcommittee of the Advisory Group on Risk Evidence Education for Dementia. Dr. Grill has served in an unpaid role on an advisory board convened by the National Institute on Aging. Ms. Ferber reports no relevant disclosures. Dr. Blacker has received consulting fees for legal consultation from Saul Ewing Arnstein & Lehr, LLP, and the estate of Barbara Bartle; and for epidemiology forecasting from Biogen (2015‐18). Dr. Grill has received consulting fees from SiteRx, Flint Rehab, and Cogniciti. Dr. Roberts has received payment from the National Institutes of Health for scientific review activities.
Figures
References
-
- Hintz EA, Dean M. Best practices for returning research findings to participants: methodological and ethical considerations for communication researchers. Commun Methods Meas. 2020;14:38‐54.
-
- Downey AS, Busta ER, Mancher M, Botkin JR. Returning Individual Research Results to Participants: Guidance for a New Research Paradigm. Washington (DC). 2018. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous