Evaporation-Based Low-Cost Method for the Detection of Adulterant in Milk
- PMID: 34693139
- PMCID: PMC8529649
- DOI: 10.1021/acsomega.1c03887
Evaporation-Based Low-Cost Method for the Detection of Adulterant in Milk
Abstract
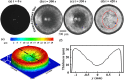
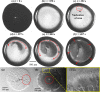
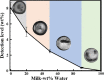
Adulteration of milk poses a severe health hazard, and it is crucial to develop adulterant-detection techniques that are scalable and easy to use. Water and urea are two of the most common adulterants in commercial milk. Detection of these adulterants is both challenging and costly in urban and rural areas. Here we report on an evaporation-based low-cost technique for the detection of added water and urea in milk. The evaporative deposition is shown to be affected by the presence of adulterants in milk. We observe a specific pattern formation of nonvolatile milk solids deposited at the end of the evaporation of a droplet of unadulterated milk. These patterns alter with the addition of water and urea. The evaporative deposits are dependent on the concentrations of water and urea added. The sensitivity of detection of urea in milk improves with the dilution of milk with water. We show that our method can be used to detect a urea concentration as low as 0.4% in milk. Based on the detection level of urea, we present a regime map that shows the concentration of urea that can be detected at different extents of dilution of milk.
© 2021 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures



References
-
- Poetschke P. The Chlorine Content of Milk. J. Ind. Eng. Chem. 1912, 4, 38–40. 10.1021/ie50037a013. - DOI
-
- Griffin M.Overview of Worldwide School Milk Programmes, 3rd International School Milk Conference, FAO, Kunming, 2005; pp 1–10.
LinkOut - more resources
Full Text Sources

