Sustainable Synthesis of Cyclic Carbonates from Terminal Epoxides by a Highly Efficient CaI2/1,3-Bis[tris(hydroxymethyl)-methylamino]-propane Catalyst
- PMID: 34693148
- PMCID: PMC8529664
- DOI: 10.1021/acsomega.1c04086
Sustainable Synthesis of Cyclic Carbonates from Terminal Epoxides by a Highly Efficient CaI2/1,3-Bis[tris(hydroxymethyl)-methylamino]-propane Catalyst
Abstract
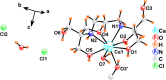
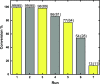
The nonstopping increment of atmospheric carbon dioxide (CO2) concentration keeps harming the environment and human life. The traditional concept of carbon capture and storage (CCS) is no longer sufficient and has already been corrected to carbon capture, utilization, and storage (CCUS). CCUS involves significant CO2 utilization, such as cyclic carbonate formation, for its cost effectiveness, less toxicity, and abundant C1 synthon in organic synthesis. However, the high thermodynamic and kinetic stability of CO2 limits its applications. Herein, we report a mild, efficient, and practical catalyst based on abundant, nontoxic CaI2 in conjunction with biocompatible ligand 1,3-bis[tris(hydroxymethyl)-methylamino]-propane (BTP) for CO2 fixation under atmospheric pressure with terminal epoxides to give the cyclic carbonates. The Job plot detected the 1:1 Ca2+/BTP binding stoichiometry. Furthermore, formation of a single crystal of the 1:1 Ca2+/BTP complex was confirmed by single-crystal X-ray crystallography. The bis(cyclic carbonate) products exhibit potentials for components in the non-isocyanate polyurethanes (NIPUs) process. Notably, this protocol shows attractive recyclability and reusability.
© 2021 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures




Similar articles
-
Efficient Synthesis of Cyclic Carbonates from Unsaturated Acids and Carbon Dioxide and their Application in the Synthesis of Biobased Polyurethanes.Chempluschem. 2021 Mar;86(3):460-468. doi: 10.1002/cplu.202100079. Chempluschem. 2021. PMID: 33704907
-
Poly(ethylene glycol)s as Ligands in Calcium-Catalyzed Cyclic Carbonate Synthesis.ChemSusChem. 2017 Aug 10;10(15):3025-3029. doi: 10.1002/cssc.201700788. Epub 2017 Jul 12. ChemSusChem. 2017. PMID: 28699190
-
Nickel(II) Complexes of Tripodal Ligands as Catalysts for Fixation of Atmospheric CO2 as Organic Carbonates.Chem Asian J. 2023 Mar 14;18(6):e202201204. doi: 10.1002/asia.202201204. Epub 2023 Feb 9. Chem Asian J. 2023. PMID: 36734191
-
Co-Catalyst-Free Chemical Fixation of CO2 into Cyclic Carbonates by using Metal-Organic Frameworks as Efficient Heterogeneous Catalysts.Chem Asian J. 2020 Aug 17;15(16):2403-2427. doi: 10.1002/asia.202000424. Epub 2020 Jul 2. Chem Asian J. 2020. PMID: 32524760 Review.
-
Synthesis of cyclic carbonates from CO2 cycloaddition to bio-based epoxides and glycerol: an overview of recent development.RSC Adv. 2023 Jul 26;13(33):22717-22743. doi: 10.1039/d3ra03028h. eCollection 2023 Jul 26. RSC Adv. 2023. PMID: 37502825 Free PMC article. Review.
Cited by
-
Can any Basic/Nucleophile Quaternary Salt Promote the Carbonatation of Epoxides? A Review.ACS Omega. 2025 Jul 24;10(33):36824-36865. doi: 10.1021/acsomega.5c03990. eCollection 2025 Aug 26. ACS Omega. 2025. PMID: 40893270 Free PMC article. Review.
-
Influence of Donor/Withdrawing Groups in an 3,5-Aryl-Substituted Pyrazole Organocatalyst for the Chemical Fixation of CO2.ACS Omega. 2025 Jun 6;10(23):24224-24234. doi: 10.1021/acsomega.4c11307. eCollection 2025 Jun 17. ACS Omega. 2025. PMID: 40547699 Free PMC article.
-
Crystal structure of the monoglycidyl ether of isoeugenol.Acta Crystallogr E Crystallogr Commun. 2022 Sep 27;78(Pt 10):1052-1055. doi: 10.1107/S2056989022009264. eCollection 2022 Oct 1. Acta Crystallogr E Crystallogr Commun. 2022. PMID: 36250116 Free PMC article.
References
-
- Lindsey R.Climate Change: Atmospheric Carbon Dioxide. https://www.climate.gov/news-features/understanding-climate/climate-chan... (accessed May 4, 2020).
-
- Peters G. P.; Andrew R. M.; Canadell J. G.; Friedlingstein P.; Jackson R. B.; Korsbakken J. I.; Le Quéré C.; Peregon A. Carbon dioxide emissions continue to grow amidst slowly emerging climate policies. Nat. Clim. Change 2020, 10, 3–6. 10.1038/s41558-019-0659-6. - DOI
-
- Lopes E. J. C.; Ribeiro A. P. C.; Martins L. M. D. R. S. New Trends in the Conversion of CO2 to Cyclic Carbonates. Catalysts 2020, 10, 47910.3390/catal10050479. - DOI
-
- Boot-Handford M. E.; Abanades J. C.; Anthony E. J.; Blunt M. J.; Brandani S.; Mac Dowell N.; Fernández J. R.; Ferrari M.-C.; Gross R.; Hallett J. P.; Haszeldine R. S.; Heptonstall P.; Lyngfelt A.; Makuch Z.; Mangano E.; Porter R. T. J.; Pourkashanian M.; Rochelle G. T.; Shah N.; Yao J. G.; Fennell P. S. Carbon capture and storage update. Energy Environ. Sci. 2014, 7, 130–189. 10.1039/C3EE42350F. - DOI
LinkOut - more resources
Full Text Sources
Miscellaneous

