Cr(VI)-Mediated Homogeneous Fenton Oxidation for Decolorization of Methylene Blue Dye: Sludge Free and Pertinent to a Wide pH Range
- PMID: 34693149
- PMCID: PMC8529654
- DOI: 10.1021/acsomega.1c04090
Cr(VI)-Mediated Homogeneous Fenton Oxidation for Decolorization of Methylene Blue Dye: Sludge Free and Pertinent to a Wide pH Range
Abstract
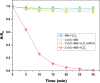
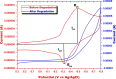
Fe(II)-mediated Fenton process is commonly employed for oxidative degradation of recalcitrant pollutants in wastewater. However, the method suffers from limitations like narrow working pH range and iron sludge formation. The present work deals with the degradation of Methylene Blue (MB) dye using Fenton-like oxidation by replacing Fe(II) with Cr(VI), which eliminates the limitations of classical Fenton oxidation. The Fenton-like oxidation of MB is brought about by HO• radicals generated by the disproportionation of chromium-coordinated peroxo complexes. It was observed that the working pH range for the Cr(VI)-mediated Fenton oxidation was 3-10, and no sludge formation takes place up to four cycles as the oxidation remains in the pure solution phase. The complete mineralization of dye was confirmed by observing the decay of MB peaks by a spectrophotometer and cyclic voltammetry. The reaction parameters like pH of the solution, temperature, degradation time, concentrations of H2O2, Cr(VI), and MB were studied for optimal performance of the Cr(VI) as the catalyst. Kinetic studies revealed that the Cr(VI)-mediated Fenton reaction follows pseudo-first-order reaction kinetics and depends on the concentration of HO• radicals. The proposed Cr(VI)-mediated Fenton oxidation in the present work is best suited for the degradation of organic dyes by adding H2O2 as a precursor in chromate-contaminated wastewaters.
© 2021 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures









References
-
- Fenton H. J. H. LXXIII. - Oxidation of Tartaric Acid in Presence of Iron. J. Chem. Soc. Trans. 1894, 65, 899–910. 10.1039/CT8946500899. - DOI
-
- Wang Q.; Tian S.; Ning P. Degradation Mechanism of Methylene Blue in a Heterogeneous Fenton-like Reaction Catalyzed by Ferrocene. Ind. Eng. Chem. Res. 2014, 53, 643–649. 10.1021/ie403402q. - DOI
-
- Espinosa J. C.; Catalá C.; Navalón S.; Ferrer B.; Álvaro M.; García H. Iron Oxide Nanoparticles Supported on Diamond Nanoparticles as Efficient and Stable Catalyst for the Visible Light Assisted Fenton Reaction. Appl. Catal., B 2018, 226, 242–251. 10.1016/j.apcatb.2017.12.060. - DOI
LinkOut - more resources
Full Text Sources
Miscellaneous

