From the niche to malignant hematopoiesis and back: reciprocal interactions between leukemia and the bone marrow microenvironment
- PMID: 34693187
- PMCID: PMC8520063
- DOI: 10.1002/jbm4.10516
From the niche to malignant hematopoiesis and back: reciprocal interactions between leukemia and the bone marrow microenvironment
Abstract
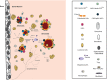
The bone marrow microenvironment (BMME) regulates hematopoiesis through a complex network of cellular and molecular components. Hematologic malignancies reside within, and extensively interact with, the same BMME. These interactions consequently alter both malignant and benign hematopoiesis in multiple ways, and can encompass initiation of malignancy, support of malignant progression, resistance to chemotherapy, and loss of normal hematopoiesis. Herein, we will review supporting studies for interactions of the BMME with hematologic malignancies and discuss challenges still facing this exciting field of research. © 2021 The Authors. JBMR Plus published by Wiley Periodicals LLC on behalf of American Society for Bone and Mineral Research.
Keywords: AGING; BONE; CANCER; CHEMOTHERAPY; HEMATOPOIESIS; HEMATOPOIETIC STEM CELL (HSC) NICHE; INFLAMMATION; LEUKEMIA; MICROENVIRONMENT.
© 2021 The Authors. JBMR Plus published by Wiley Periodicals LLC on behalf of American Society for Bone and Mineral Research.
Figures
References
-
- Till JE, McCulloch EA. A direct measurement of the radiation sensitivity of normal mouse bone marrow cells. Radiat Res. 1961;14(2):213‐222. - PubMed
-
- Schofield R. The relationship between the spleen colony‐forming cell and the haemopoietic stem cell. Blood Cells. 1978;4(1‐2):7‐25. - PubMed
-
- Lord B, Testa N, Hendry J. The relative spatial distributions of CFUs and CFUc in the normal mouse femur. Blood. 1975;46(1):65‐72. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
